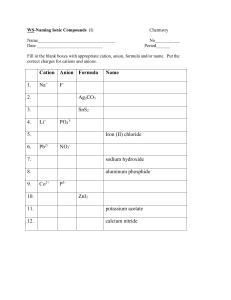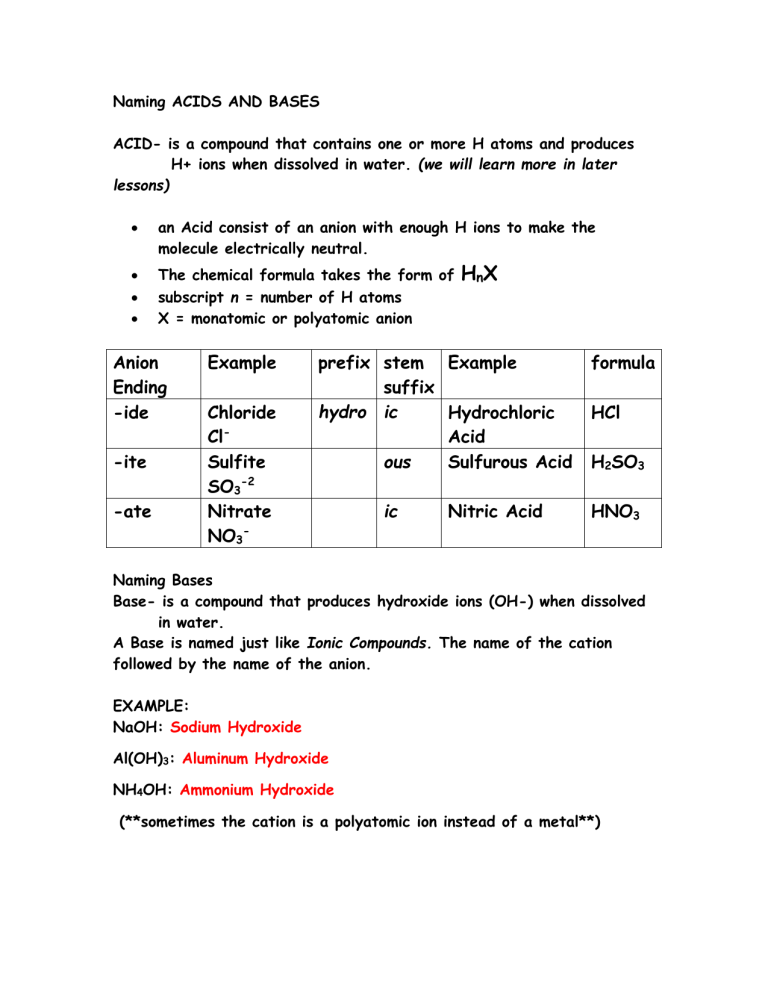
Naming ACIDS AND BASES ACID- is a compound that contains one or more H atoms and produces H+ ions when dissolved in water. (we will learn more in later lessons) an Acid consist of an anion with enough H ions to make the molecule electrically neutral. The chemical formula takes the form of subscript n = number of H atoms X = monatomic or polyatomic anion Anion Ending -ide -ite -ate Example Chloride ClSulfite SO3-2 Nitrate NO3- HnX prefix stem Example suffix hydro ic Hydrochloric Acid ous Sulfurous Acid ic Nitric Acid formula HCl H2SO3 HNO3 Naming Bases Base- is a compound that produces hydroxide ions (OH-) when dissolved in water. A Base is named just like Ionic Compounds. The name of the cation followed by the name of the anion. EXAMPLE: NaOH: Sodium Hydroxide Al(OH)3: Aluminum Hydroxide NH4OH: Ammonium Hydroxide (**sometimes the cation is a polyatomic ion instead of a metal**)