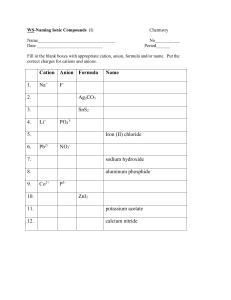
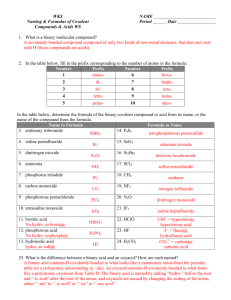
Naming Acids, Bases, and Salts Chemistry Worksheet
advertisement
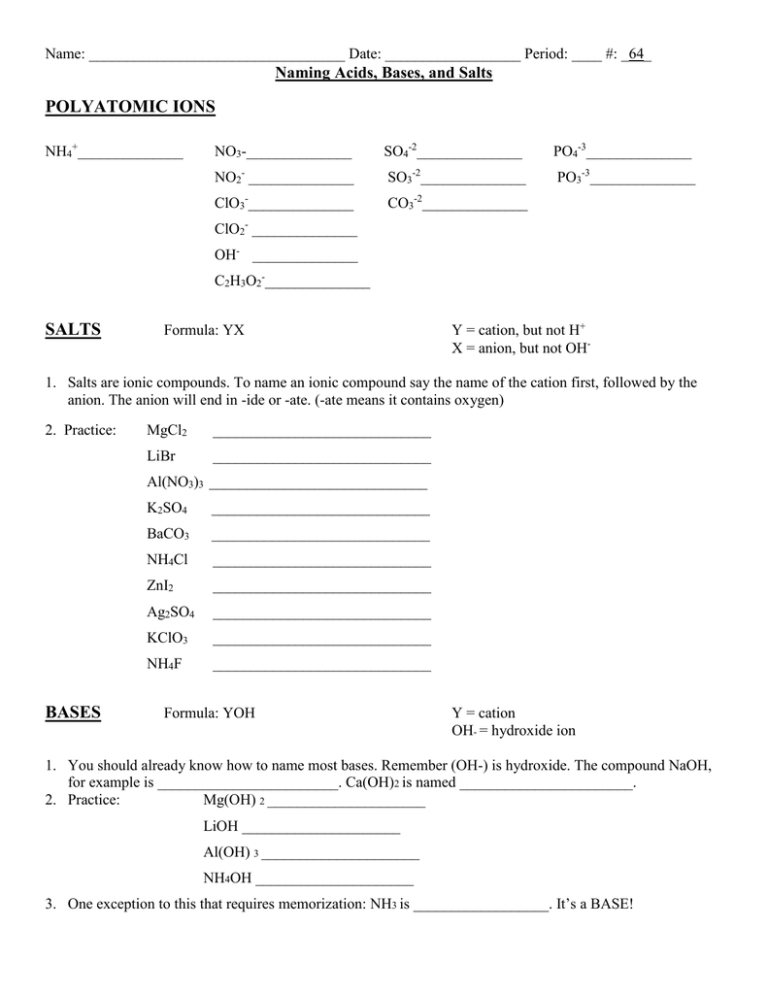
Name: __________________________________ Date: __________________ Period: ____ #: _64_ Naming Acids, Bases, and Salts POLYATOMIC IONS NH4+______________ NO3-______________ SO4-2______________ PO4-3______________ NO2- ______________ SO3-2______________ PO3-3______________ ClO3-______________ CO3-2______________ ClO2- ______________ OH- ______________ C2H3O2-______________ SALTS Formula: YX Y = cation, but not H+ X = anion, but not OH- 1. Salts are ionic compounds. To name an ionic compound say the name of the cation first, followed by the anion. The anion will end in -ide or -ate. (-ate means it contains oxygen) 2. Practice: MgCl2 _____________________________ LiBr _____________________________ Al(NO3)3 _____________________________ BASES K2SO4 _____________________________ BaCO3 _____________________________ NH4Cl _____________________________ ZnI2 _____________________________ Ag2SO4 _____________________________ KClO3 _____________________________ NH4F _____________________________ Formula: YOH Y = cation OH- = hydroxide ion 1. You should already know how to name most bases. Remember (OH-) is hydroxide. The compound NaOH, for example is ________________________. Ca(OH)2 is named _______________________. 2. Practice: Mg(OH) 2 _____________________ LiOH _____________________ Al(OH) 3 _____________________ NH4OH _____________________ 3. One exception to this that requires memorization: NH3 is __________________. It’s a BASE! ACIDS Formula: H X H+ = hydrogen ion X = anion 4. BINARY ACIDS RULE says: If the anion ends in –ide, hydro ______________ic acid Example: HCl hydrochloric acid Practice: HBr _____________________ HI _____________________ HF ______________________ 5. TERNARY ACIDS RULE 1 says: If the polyatomic anion ends in –ate Hydro ______________ic acid Example: HClO3 chloric acid Practice: HNO3 _____________________ H2CO3 _____________________ H2SO4 _____________________ HC2H3O2 ______________________ H3PO4 ______________________ 6. TERNARY RULE 2 says: If the polyatomic anion ends in –ite Hydro ______________ous acid H2SO3 ______________________ H2ClO2 ____________________ H3PO3 ______________________ HNO2 ______________________