Sheet-1
Chapter 04: ELECTROCHEMISTRY
Electrochemistry: The branch of chemistry that deals with the chemical change associated with electrons and
electricity is called electrochemistry. In other words, the branch of chemistry that deals with the interconversion
of electrical energy and chemical energy is called electrochemistry.
Electrical Conduction and Electrical Conductivity:
The process by which a substance conducts electricity from one place to another is called electrical conduction
and the ability of a substance to conduct an electric current is called electrical conductivity. Based on electrical
conductivity substances are classified into two classes: (1) Conductor or Electrical conductor (2) Insulator.
(1) Conductor or Electrical conductor: The substances/materials which can conduct electricity are called
conductors/electrical conductors. For example, all metals (like Fe, Cu, Ag etc.), graphite carbon, molten salts,
solutions of salt, acid and base.
Again the substances which allow the passage of electricity with greater ease are called good conductors as
for example Fe, Cu, Ag etc. Those substances which can conduct electricity with lesser ease are called poor
conductors. Water is a poor electrical conductor.
(2) Insulator or Non-conductor: The substances/materials which cannot conduct electricity are called nonconductors/insulators. For example, non metals (except graphite carbon), glass, hydrocarbons like kerosene,
petrol, benzene etc. In fact there is no matter which completely acts as a non-conductor. The magnitude of
conductance in some substances is so little that they are called nonconductors.
Types of electrical conductors: On the basis of mechanism of electrical conductivity, the conductors are of two
types: (1) Electronic conductor or metallic conductor and (2) Electrolytic conductor or electrolyte.
(1) Electronic conductors or Metallic conductors or Non-electrolytic conductors: The conductor which
passes electricity through electron flow is called electronic conductor. In other words, the conductors which
carry electric charge by their mobile electrons and no chemical change occurs during the passage of electricity
through them are called electronic conductors. Example: all metals, graphite, metal alloys and some metallic
oxides. Electronic conductors are of three types: (i) Good conductor (ii) Semiconductor or bad conductor (iii)
Super conductor
(i)
Good conductor: The conductors that conduct electricity easily are called good conductors. e.g Cu, Al.
(ii)
Semiconductors or bad conductors: The conductors which can conduct electricity partially are called
semi-conductors. In other words conductors which have an electrical conductivity between that of a good
conductor and an insulator are called semi-conductors. For example Si, Ge, Ga etc.
(iii)
Super conductor: The conductors which can conduct electricity with no resistance are called super
conductors. Examples are metal alloy and oxides of metal alloy. Each of the super conductors has a
temperature below which super conductor can conduct electricity without any resistance; this temperature
is called super conducting transition temperature (Tc). For example Nb3Ge-alloy has Tc= 23.2K and Tc
YBa2Cu3O7-alloy has Tc= 90K.
(2) Electrolytic conductors or Electrolytes: The conductor which passes electricity through ions of the
conductor is called electrolytic conductor. In other words, the compounds (ionic or polar) which conduct
electricity in molten states or in solutions by their mobile ions accompanied by the chemical changes during the
flow of electricity are called electrolytes. Example: all ionic compounds, molten salts, solutions of salt, acid and
base etc. Electrolytes are of two types: (i) Strong electrolytes, (ii) Weak electrolytes.
(i)
Strong electrolytes: The compounds which dissociate into ions in solutions almost completely and can
conduct electricity in high degree are called strong electrolytes. Examples are the solutions of NaCl,
NaOH, HC1 etc.
(ii)
Weak electrolytes: The compounds which dissociate into ions in solutions very poorly and can conduct
electricity in fewer amounts are called weak electrolytes. Examples are solutions of NH3, CH3COOH,
H2CO3, HCN, ZnCl2 etc.
**Non-electrolyte: The compounds which do not conduct electricity in molten states or in solutions are called
non-electrolytes. Non polar covalent compounds like hydrocarbons, CCl4, glucose, sugar solutions etc. are nonelectrolytes.
Table: Difference between electronic conductors and electrolytes
Subject
Electronic or metallic conductor
Electrolyte
1. Conduction In electronic conductors, current is In electrolytes, current is conducted by movement of
by:
conducted by movement of electrons cations and anions towards the cathode and anode
without actual transfer of matter.
respectively with transfer of matter.
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
1
2. Example
3. Physical
state
4. Capacity
5. Chemical
Change
6.
Temperature
7. Applicable
Law
All metals, graphite, metal alloys and
some metallic oxides.
Electronic conductors conduct electricity
in solid state.
Metallic conductor has a high capacity of
conducting electricity.
No chemical change occurs during
electronic conductance of electricity; only
they are heated.
With the increase of temperature the
conductivity of metal decreases.
Ohm's Law is followed by metallic
conductors.
All ionic compounds, molten salts, solutions of salt,
acid and base etc.
Electrolytes conduct electricity in solution or molten
state.
The current carrying capacity of a particular
electrolyte is limited.
Chemical changes occur during passage of
electricity through electrolytes at anode and
cathode.
With the increase of temperature the conductivity of
electrolyte increases.
Faraday's Laws are followed by electrolytes.
Some terms used in electrical measurements
The metallic and nonmetallic electric conductors (called electronic conductors) which connect the electronic
conductor and solution (which convey ions) of electrochemical cell are called electrodes. Two electrodes are
needed to construct an electrochemical cell. One is anode and another is cathode.
Cathode: In electrochemical cell the electrode which donates electron to electrolytic substance is called cathode.
Important features of cathode are: (i) In cathode reduction reaction occurs (e.g. Mn+ + ne- → M) (ii) In
electrolytic cell cations of solution accept electrons from metallic rod (Cathode) (iii) In galvanic cell cathode is
positive but in electrolytic cell cathode is negative.
Anode: In electrochemical cell the electrode which accepts electron from electrolytic substance is called anode.
Important features of anode are: (i) In anode oxidation reaction occurs (e.g. M → Mn+ + ne-), (ii) In
electrolytic cell electrons of anion of solution transfer to metallic rod (Anode), (iii) In galvanic cell anode is
negative but in electrolytic cell anode is positive.
***Classification of electrode will be discussed later.
Electric Charge: The SI unit of electric charge is coulomb and its symbol is C. The amount of electric charge
which passes through a conductor, when 1 ampere current is passed for 1 second is called a coulomb.
Coulomb (c) = ampere (A) ×second (s). Again, 96500 coulomb = 1 Faraday.
Electric current: The rate of passage of electric charge through a conductor is called electric current. Its unit is
ampere and its symbol is A.
Coulomb (C)
= Cs─1
Therefore, Ampere (A) =
Second (s)
Electric potential: The amount of work which is done to bring a unit positive charge from infinity to a point in
an electric field is called electric potential at that point. Its unit is volt and symbol is V.
Work done(J)
=JC─1
Electric potential (V) =
Amount of charge (C)
Electrolysis
The process of chemical decomposition of an electrolyte in molten stage or in solution by passing electricity
through it is called electrolysis.
Mechanism of electrolysis: When electricity is supplied to the electrolytic cell there creates a positive pole
electrode (anode) and negative pole electrode (cathode). For this reason the ion present in electrolytic solution
are attracted by the electrode according to their charge. That means the negative ion is attracted by the anode and
the positive ion is attracted by the cathode. Negative ions release electron to anode (oxidation) and form new
substance. On the other hand positive charge ions accept electron from cathode (reduction) and form new
compound. In this way in electrolytic cell the produced electron in anode by the oxidation reaction pass through
the cell to the cathode to meet the demand of electron for reduction reaction.
Anode reaction:
X─ → X + e ─
or,
Xn─ → X + ne─
Cathode reaction:
M++ e─→ M
or,
Mn++ ne─→ M
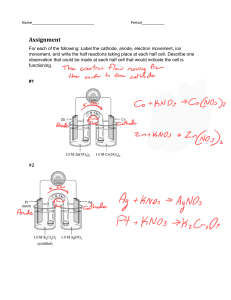
Q. Electrolysis is an Oxidation-Reduction Process-Explain
According to modern definition, oxdation is a chemical process, in which an ion or atom donates one or more
electrons and reduction is a chemical process, in which an ion or atom accepts one or more electrons.
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
2
During electrolysis cation accepts one or more electrons from the cathode and is reduced. On the other hand
anion donates one or more electrons to the anode and is oxidized. For example, during the electrolysis of molten
sodium chloride (NaCl) following processes take place at the two electrodes:
2Na+(l) + 2Cl─(l)
2NaCl(l)
Anode reaction:
2Cl─(l)→Cl2(g)+2e─[Oxidation]
Cathode reaction:
2Na+(l) + 2e─ → 2Na(s) [Reduction]
In fact in all electrolysis reduction takes place at the cathode and oxidation at the anode. Therefore electrolysis is
an oxidation-reduction process.
Some Examples of Electrolysis are described below:
(1) Electrolysis of molten NaCl : When electricity is passed through fused or molten NaCl,
chlorine gas is liberated at the anode and sodium metal is deposited at the cathode.
2NaCl(l) → 2Na+(l) + 2Cl─(l)
At anode :
2Cl─(l) → Cl2(g) + 2e─
At cathode : 2Na+(l) + 2e─ → 2Na(s)
(2) Electrolysis of Sodium Chloride Solution: Saturated aqueous solution of sodium chloride
is called brine. In the electrolytic cell the anode and cathode are dipped in aqueous solution of
NaCl (brine). The sodium chloride solution contains Na+, Cl─, H+ and OH─ ions i.e. two
cations and two anions. When electricity is passed through the NaCl solution, Cl─ ions are
oxidized to Cl2-gas in anode and H+ ions are reduced to H2- gas in cathode.
Cations: Na+,H+
Anode reaction:
2Cl─(aq)→Cl2(g) + 2e─
[Oxidation]
Anions: Cl─,OH─
Cathode reaction: 2H+(aq) +2e─→H2 (g)
[Reduction]
─
+
Overall reaction: 2Cl (aq) + 2H (aq) →Cl2(g)+ H2 (g) [Redox]
In fact electrons produced in anode reach to cathode by wire and supply the required electron for reduction of
water. Hydroxyl ions (OH─) produced in cathode and join together with sodium ion and remain in solution as
NaOH. Thus NaOH is found as by-product with chlorine and hydrogen gas on electrolysis of sodium chloride
solution. [Note: Why do Na+ and OH─ ions not move to electrode that can be explained with the help of E.C.S]
(3) Electrolysis of Water (mixed with a small amount of acid like H2SO4):
Making some acidic solution of water with a small quantity of H2SO4 and supplying electricity by Platinum
anode and cathode, the electrolysis of water is done. Hydrogen ion (H+) and hydroxyl ion (OH─) are produced
by the dissociation of water. In cathode H+ ion is reduced to H2 and in anode OH─
ion is oxidized to O2.
Cations: H+
+
─
Anions: OH─, SO4─
Dissociation of water: H2O(l)
H (aq)+OH (aq)
Anode reaction: 4OH─(aq) → O2(g) + 2H2O+4e─ [Actually: 2O2─ → O2+4e─]
Cathode reaction: 4H+(aq)+4e─→ 2H2(g)
.
Overall reaction: 4H2O(l) → 2H2(g) + O2(g) + 2H2O(l)
Or,
2H2O(l) → 2H2(g) + O2(g)
Q. Why is H2SO4 mixed with water during the electrolysis of water?
Ans: Actually during the electrolysis of water no change of sulfuric acid takes place, it only acts as conveyer of
electricity through the solution. Pure water is a bad conductor of electricity or sometimes it does not conduct
electricity. When some acid like H2SO4 is mixed with water it becomes good conductor of electricity. The
added acid also helps the ionization of the water. For this reason H2SO4 mixed with water during the electrolysis
of water.
(4) Electrolysis of copper sulphate solution:
CuSO4(aq) → Cu2+(aq) + SO42─(aq)
Cations: Cu2+, H+
H2O(l)
H+(aq) + OH─(aq)
Anions: OH─, SO4─
Cathode reaction: Cu2+(aq) +4e─→ Cu(s)
Anode reaction: 4OH─(aq)→2H2O(l) + O2(g) + 4e─
[Actually: 2O2─ → O2+4e─]
Overall reaction: Cu2+(aq) +4OH─(aq)→ Cu(s) + 2H2O(l) + O2(g)
Electrochemical Series (E.C.S):
The lists of cations and anions in order of their ease of discharge at the electrodes are called the electrochemical series. There are two series; one for cations or positive ions and the other for anions or negative ions
according to their reduction potential (for cations) and oxidation potential (for anions). When an electron is
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
3
accepted by a cation energy is released, it is called reduction potential and when an electron is released by an
anion, energy is absorbed, it is called oxidation potential. They are also called electrode potentials. Thus E.C.S
may also be defined as follows:
The list of cations that has been made on increasing order of their reduction potential and the list of anions that
has been made on increasing order of their oxidation potential are collectively known as electrochemical series.
Table-1: Electrochemical series (partly)
Characteristics of E.C.S:
(i)
Relative ability to be discharged: The electrochemical series reflects the reactivity of metal ions to be
reduced, which is opposite to the reactivity of the metal atoms. For example, K atom is more reactive
than Na atom, but on electrolysis of their ions opposite order of reduction prevails i.e. first discharge of
Na+ ion and then K+ ion occurs.
Na+ + e→Na.
(ii)
Influence of concentration of ions on E.C.S: When the concentration of one ion is very much greater
than that of another ion, then the order of ions in E.C.S is disturbed, to be discharged. For example, the
position of OH─ ion is below Cl─ ion in E.C.S. So when a dilute solution of NaCl is electrolysed, then
OH─ ion is discharged first and not Cl─ ion. But when a concentrated solution of NaCl, say brine, is
electrolysed, the concentration of Cl─ ion is much more than that of OH─ ion; so in this case Cl─ ion is
discharged and not OH─ ion.
(iii)
Nature of the electrodes and behaviour towards cations: The nature of the electrodes also plays an
important role in leading which cation between the two is to be discharged first. For example when an
aqueous solution of NaCl is electrolysed with two inactive platinum electrodes H+ is discharged at the
cathode with evolution of H2 gas and not Na+ ions. But when mercury is used as cathode, metallic Na is
formed by discharging of Na+ ion with the formation of sodium mercury amalgam (Na-Hg).
Faraday's Laws of Electrolysis
In 1833 Michael Faraday discovered two laws regarding amount of electricity passed through an electrolyte and
the amount of chemical changes occured at the electrodes. These two laws are called Faraday's Laws of
electrolysis. Faraday's Laws are treated as: (1) Faraday's First Law and (2) Faraday's Second Law. Only
Faraday's First Law is included in syllabus and hence is discussed below:
(1) Faraday's First Law: ‘The mass of any substance deposited or dissolved at any electrode during electrolysis
is directly proportional to the amount of electricity passed through the electrolyte’.
Explanation: If Wg of a substance dissolved or deposited at an electrode due to the passage of Q coulomb
electricity through the electrolyte, then according to Faraday's first law:
W Q
W It
[ Q = It; where, I = amount of current in ampere t = time is seconds]
W = ZIt,
Here, Z is known as electrochemical equivalent of the element When, I=1 unit say 1 ampere, t = 1 second, then
W = Z; Therefore the electro chemical equivalent (E.C.E) of an element can be defined as the amount of the
substance deposited or dissolved when a current of 1A is passed through ions of that element for 1 second.
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
4
For example: The electrochemical equivalent of H is 0.000010447 gC─1, the E.C.E of Cu is 0.000329 gC─1.
Electro chemical equivalent (Z) of any element can be calculated using the following equation:
Atomic mass of the element
Atomic mass of the element
Electro chemical equivalent (Z) of any element =
or
n×F
Valency×96500
Where, n=Number of electrons donated or gained and F=Faraday’s constant =96500
[Note: Chemical Equivalent: The amount of element deposited or dissolved when 1F (i.e. 96500C) charge is
passed through the ions of that element is called the chemical equivalent of that element]
Experimental Proof: We know, when AgNO3 solution is electrolysed, silver ion (Ag+) is reduced at cathode:
Ag+(aq) + e─→Ag(s)
1mol 1mol 1mol
From the above equation it is clear that 1 mole silver ion is reduced by 1 mole
electron to produce 1 mole silver atom. Again, amount of produced silver is
proportional to number of electrons passed in the electrolyte i.e. proportional to the
amount of current passed through the circuit. Again 1 mole of Ag = 108g and it
contains Avogadro number (NA = 6.022×1023) of silver atoms. But 1mole of electrons
also has same number of electrons and charge of 1 electron is 1.602×10─19 coulomb.
Therefore total charge of 1 mole of electrons = 1.602×10─19×6.022×1023C = 96473 C
= 96500C (approx). This amount of electricity is known as one Faraday and it is denoted by F.
1 F = 96500 C
i.e. 1F electric charge is required to produce 1 mole silver (Ag).
n × F ×(electric charge) is required to produce n mole silver (Ag).
Therefore, amount of silver deposited at electrode is proportional to amount of electric charge. This is Faraday's
First Law of electrolysis.
Faraday constant: From Faraday's First Law, we can know that 1 mole electrons have 96500 C charges. The
amount of 96500 coulomb per mole electric charge (Cmol─1) is known as Faraday constant.
1 F = 96500 coulombs.
(2) Faraday's Second Law: If same amount of electricity is passed through different electrolytes, then the
amount of substances deposited or dissolved at different electrodes will be proportional to their respective
chemical equivalent. Faraday's Second Law can also be stated as follows:
If same amount of electricity is passed through different electrolytes, then the amount of substances deposited or
dissolved at different electrodes will be proportional to their atomic masses divided by their respective valency
or charge number of the ions. [N.B: Second law is not included in syllabus; see the statement only]
Applications of Faraday's First Law:
i. By Applying Faraday's First Law the amount of different substances that are deposited at the electrodes by
the passage of definite amount of electricity can be calculated.
ii. By Applying Faraday's first law the electrochemical equivalent (E.C.E) of an element can be calculated.
iii. The charge of an electron can be calculated with the help of Faraday's law:
From Faraday's First Law we know: NA × e─ = 1 Faraday = 96500 C;
Here, e─ = charge of an electron, NA = Avogaro’s number.
NA ×e─ = 96500 C
96500C
96500C
Or, e─ =
=
=1.602×10─19C
6.022×1023
NA
The amount of charge calculated from Faraday's Law is equal to charge of electron obtained from different
experiments. From this fact, it is proved that 1 mol electron = 1 Faraday.
Limitation of Faraday's First Law:
(i)
Faraday's Laws are applicable to electrolytic conductors but not to electronic conductors where chemical
reactions do not occur.
(ii)
This Law can be applied quantitatively where 100% electricity is conducted and utilized for electrolysis
and not for ionisation of electrolytes.
(iii)
This Law can not be applied quantitatively where more than one reaction occur in electrolysis.
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
5
Validity of Faraday's Law: The temperature of electroyte, pressure, dissolved solvent and concentration of
solution have no effect on Faraday’s law e.g. at 20°C temperature, if 2.5A current is passed through 1M AgNO3
solution, the amount of Ag deposited will be equal to the amount when same amount of current will be passed
through AgNO3 solution at 25°C having concentration 5M.
*** Solve Faraday's First Law related mathematical problems from mathematical problem section-(A)
Reactivity/Electropositivity/Reducing property increasing
Reactivity Series of Metals
The elements which can donate/release electron forming cation are called metals. The ability of a metal to
donate/release electron is called the reactivity of that metal. The electron releasing ability of all metals is not
the same. Some metals release electron quickly and some metals release slowly. The higher the electron
releasing ability of a metal the higher is its reactivity.
The series of metals obtained by placing the more reactive metals on top and less reactive metals serially below
is called the reactivity series of metals. In other words, reactivity series of metals is that series in which metals
are arranged in the decreasing order of their reactivity. A metal can replace another metal located below of that
metal in the series. For example, the metals located above hydrogen can replace hydrogen from acid. Reactivity
series some important metals are shown below:
Metal Symbol Oxidation Potential
Reactivity
potential
Lithium
Li
+3.045
Replaces H from water, steam and acid and forms
Potassium K
+2.925
hydroxide.
Calcium
Ca
+2.87
Sodium
Na
+2.714
Magnesium Mg
+2.36
Replaces H from steam and acid and forms hydroxide.
Aluminium Al
+1.66
Carbon
C
Zinc
Zn
+0.763
Chromium Cr
+0.74
Iron
Fe
+0.44
Only replaces H from acid and forms hydroxide.
Cadmium Cd
+0.40
Cobalt
Co
+0.28
Nickel
Ni
+0.25
Tin
Sn
+0.136
Lead
Pb
+0.126
Hydrogen H
0.000
Included for comparison.
Antimony Sb
Forms oxide combining with oxygen and cannot replace
H.
Arsenic
As
Bismuth
Bi
Copper
Cu
─0.337
Mercury
Hg
─0.79
Found in free state in nature; their oxides decompose on
Silver
Ag
─0.799
the application of heat.
Paladium Pd
Patinum
Pt
Gold
Au
─1.50
This table also indicates the reducing power of the metals. The reducing power decreases from top to bottom.
Therefore, lithium is the strongest reductant.
Q. Why is hydrogen placed in the reactivity of metals?
Ans: Though hydrogen is not a metal, it has been given position in the reactivity series of metal. Because
hydrogen can turn into electropositive H+ ion by releasing 1 electron. Besides the reactivity of metals can be
compared with respect to hydrogen i.e. hydrogen can be considered as the standard.
Q. Why is carbon placed in the reactivity of metals?
Ans: Like hydrogen carbon is also given position in the reactivity series of metal for comparison of the reactivity
of the other metals. Carbon can reduce the metals below it and hence these metals can be extracted from their
ores by carbon reduction process.
Oxidation Half Reaction and Reduction Half Reaction: Already discussed in chapter-3 (Quantitative
Chemistry)
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
6
Electrode Potential or Half Cell Potential
When a metal is immersed in a solution containing its own ions (say Zn in ZnSO 4 solution) then a potential
difference is produced in between the metal and its solution. This potential difference is called electrode potential
or single electrode potential. [Note: When a metal is immersed in a solution containing its own ions then a half
cell formed & it is known as metal/metal ion electrode.]
Explanation: When a strip of metal is partially immersed in aqueous solution of its own ion then the metal atom
shows tendency to go into solution as positive ions leaving its valence electrons on the surface of that metal.
M(s) →Mn+(aq) + ne─
As a result of this process, the metal strip will become negatively charged with
excess
electrons
(Fig).
n+
Again, metal ions (M ) in solution show tendency to take up electrons from the
metal strip and get discharged into metal atoms as follows:
Mn+(aq) + ne─ →M(s)
An equilibrium exists in between the two processes: M(s)
Mn+(aq) + ne─
(i)
As a result of these two opposite processes like dissolving metal atoms
into ions and discharging ions into metal atoms, an electrical potential difference is set up between metal
and its ions in solution. This electrical potential difference is known as single electrode potential.
(ii)
Every metal shows unequal different tendency to lose valance electrons to go to its solution as cations
and back to gain electrons to be reduced as metal atoms.
(iii)
According to reactivity more metal ions in solution may manage to take up
electrons from the strip of metal and be discharged as metal atoms. In this
case the metal will become positively charged (Fig)
(iv)
Two electrodes such as zinc electrode and copper electrode provide
different tendencies to release electrons. Since Zn has a higher tendency
than Cu, electron density on Zn will be higher. If two such systems of
'metal-metal ion electrodes' are connected by porous partition or salt
bridge, an electron pressure difference or a potential difference between two electrodes is established.
This difference of potential is called cell potential or e.m.f of the cell.
Oxidation Potential and Reduction Potential: Cell Potential or E.M.F of Cell: The tendency of the metal of a
half cell to form metal ion is called its oxidation potential and the tendency of metal ion of a half cell to be
reduced and deposited as metal atom is called its reduction potential. The difference between the oxidation
potential or reduction potential of two electrodes of a cell is called cell potential or e.m.f of cell. Since at the
anode oxidation and at the cathode reduction take place hence the summation of oxidation potential of anode and
reduction potential of cathode is also called cell potential. It should be noted that oxidation potential of an
electrode is numerically equal to its reduction potential but the sign is opposite to each other. Therefore,
Ecell = Ecathode(red) + Eanode(ox) = Ecathode(red) ─ Eanode(red)= Eanode(ox) ─Ecathode(ox)
Standard Electrode Potential, Eθ
If the metal of the half cell is immersed in a solution of its ions of 1molar concentration at 25°C and for H 2 gas at
1atm pressure, then the electrode is called standard electrode and the potential of the electrode is called
standard electrode potential (Eθ). It is not possible to measure potential of a single electrode directly; because a
half cell may have potential but can not have e.m.f value; but a complete cell may have e.m.f value.
For this reason, the potential of Hydrogen electrode is taken to be equal to zero (0) and it is used as reference
electrode to form a cell with experimental electrode. The e.m.f of this cell is taken as standard electrode potential
of that experimental electrode. The magnitude of electrode potential depends on: (i) Nature of metal or ions in
solution, (ii) concentration of ions in solution, (iii) Temperature, (vi) Pressure (in Hydrogen electrode).
Electrode Potential and Reactivity Series & Electro-chemical Series:
Reactivity series is the list of metals based on the decreasing order of their oxidation potential and electrochemical series is the list of ions based on the increasing order of their reduction potential. A combined form of
reactivity series and electro-chemical series along with oxidation & reduction potentials is shown in the
following table:
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
7
Following points can be noted from standard electrode potentials (E°Ox or E°Red):
(i)
Larger values of the standard oxidation potential (E°Ox) or lower values of the standard reduction
potential (E°Red) indicate greater tendency of the metal to lose electrons i.e. higher metallic or electropositive character.
(ii)
A negative E°Red value [e.g. E°Li+/Li =-3.04V] indicates that the metal has a greater tendency to lose
electron i.e. to be oxidized into its own ion than the tendency of its ion to be reduced by gaining electron.
(iii)
A positive E°Red value indicates that the tendency of the metal ion to be reduced by gaining electron is
more than the tendency of the metal to be oxidized by losing electron,
(iv)
An element having higher negative E°Red value will replace all others below it with lower negative
potential values from their aqueous salt solution. Thus Zn will replace Cu 2+ from an aqueous solution of
CuSO4 i.e. Zn will reduce Cu2+and itself will be oxidized.
(v)
The higher the E°Red value of an element the stronger is the oxidizing power of its oxidized form and
weaker is the reducing power of its reduced form.
(vi)
An oxidant with higher E°Ox value can oxidize a system with lower E°Red value. Thus the numerical value
of E°Red is a measure of the strength of an oxidant. All oxidants are characterized by high value of E° Red
e.g. since E°F2/2F- = 2.87 V, F2 is one of the strongest oxidant and no common oxidants are known which
can oxidize fluoride ion (F─) to fluorine gas (F2).
(vii) All half cell reactions are reversible, Zn2+ + 2e─
Zn; Hence the half cell potential of any oxidation is
equal in magnitude but opposite in sign to that of the reverse process i.e. reduction. For example,
Zn - 2e─→Zn2+ (Oxidation) E°Ox = + 0.76V
Zn2+ + 2e─→Zn (Reduction) E°Red = ─ 0.76V
That is for any particular system [Mn+ + ne─ M]: E°Ox = ─ E°Red Or, E°Red = ─ E°Ox
Applications of electrode potential: The important applications of electrode potential are:
(1) Measurement of standard potential of electrodes.
(2) Calculation of E.M.F of a cell with standard electrode potential.
(3) To determine the spontaneity of a cell reaction and to identify anode and cathode of a cell.
(4) To determine the pH of an acid solution.
(5) To control the corrosion of metal by electrode potential.
Reference Electrode
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
8
The electrode of known potential by which the unknown potential of one electrode can be determined
experimentally is called reference electrode. Reference Electrodes with standard potential value are of two type:
(1) Primary Reference Electrode: The electrodes which can be used directly to determine the potential of any other
electrode are called primary reference electrode. For example, H-electrode.
(2) Secondary Reference Electrode: The electrodes which are used in common practical works after determining
their potential with the help of primary reference electrode i.e. H-electrode are called secondary reference electrode.
For example, (i) Calomel Electrode (ii) Silver-silver chloride electrode etc.
Hydrogen Electrode: The special gaseous electrode formed by passing pure H2 gas under 1 atm pressure
through the solution of H+ ions of unit activity like 1M HC1 solution at 25°C, in which Pt-foil is dipped partially
as H2 gas absorbent, is called Hydrogen electrode. The potential of H-electrode is arbitrarily taken as zero. The
H-electrode is represented as follows:
Pt, H2(g) (1atm) / H+(aq) (1M); E°=0.0V
Or,
Pt, H2(g) (1atm) / H+(aq) (1M HCl); E°=0.0V
Limitations of H-electrode: The major difficulties of H-electrode are- (i) It is very difficult to keep a constant 1
atm. pressure of H2 gas at the surface of the electrode through out the experiment, (ii) It is also difficult to
maintain 1M concentration of H+ ions in the half cell, (iii) The impurities present in the system also decrease the
activity of platinum foil.
Calomel Electrode: The most commonly used secondary reference electrode is the Calomel Electrode. Standard
Calomel Electrode is substantial electrode which is made of mercury, mercurous chloride (Hg2Cl2) or calomel
and KC1 solution. The calomel electrode is expressed as follows: Hg/Hg2Cl2(s),KCl(aq)(lM)
Cell reaction:
At anode:
2Hg(l)
+ 2Cl─
Hg2Cl2(s) + 2e─
At cathode: Hg2Cl2(s) + 2e─
2Hg(l)
+ 2Cl─
Nernst Equation Related to Electrode and Cell Potentials
The electromotive force of an electrochemical cell depends on the active mass (concentration or partial pressure)
of reactants and products of the cell reaction, temperature and the current flow. Let, the following reversible
reaction takes place in an electrochemical cell:
xA(s) + yB+ (aq)
xA+ (aq) + yB (s)
In 1889, German chemist Walther Hermann Nernst established a relation between non-standard state cell
potential (Ecell) and standard state cell potential (E°cell) for the electromotive force of above type of cell reaction.
RT [A + ]x
That equation is:
Ecell = E°cell ─
ln
nF [B+ ]y
[At standard condition active mass of solid substance one, Therefore, concentrations of ions of product and
reactant have been considered here]
In the above equation, Ecell = Cell potential (at non-standard state) i.e. non-standard cell potential; E°cell = Cell
potential (at standard state) i.e. standard cell potential; T = Absolute temperature; F = Current flow (Faraday) =
96500C; R=Universal gas constant=8.314JK─1mol─1; n = Mole number of transferred electron in the reaction;
[A+] = Concentration of product ion; [B+] = Concentration of reactant ion.
For example, in case of Daniel cell, we have,
At anode,
Zn → Zn2+ + 2e─
At cathode,
Cu2++ 2e─→ Cu
Cell reaction or overall reaction, Zn + Cu2+→ Zn2+ + Cu
Now the non-standard state zinc electrode and copper electrode potentials are related to their standard state
potentials as follows:
2+
RT Zn
RT
2+
2+
ln
ln Zn 2+ ---------------------(i) [ [Zn(s)]=1]
EZn/Zn = E°Zn/Zn ─
= E°Zn/Zn2+ ─
nF
nF
Zn
ECu2+/Cu = E°Cu2+/Cu ─
Now, cell potential,
RT
nF
ln
Cu
Cu
2+
= E°Cu2+/Cu +
RT
nF
ln Cu 2+ --------------------(ii) [
[Cu(s)]=1]
Ecell = Ecathode(red) + Eanode(ox)
Or, Ecell = {E°Cu2+/Cu +
RT
nF
ln[Cu 2+ ] } + { E°Zn/Zn2+ ─
Or, Ecell = {E°Cu2+/Cu + E°Zn/Zn2+} + {
RT
nF
ln[Cu 2+ ] ─
RT
nF
RT
nF
ln Zn 2+ }
ln[Zn 2+ ] }
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
9
Or, Ecell = {E°Cu
2+
/Cu +
E°Zn/Zn
2+
Cu 2+
ln
}+
nF Zn 2+
RT
Zn 2+
A
B
ln
Or, Ecell = E°cell ─
[ E°cell =E°cathode(red) + E°anode(ox) & ln = ─ ln ]
2+
nF Cu
B
A
RT
Or, Ecell = E°cell ─
2.303RT
nF
Zn 2+
log
--------------------(iii)
Cu 2+
If the cell reaction is carried out at 25°C temperature then T=298K, R=8.314JK─1mol─1, F=96500C, and then
from equation (iii) we have,
Zn 2+
Zn 2+
2.303 8.314 298
0.0592
log
log
Ecell = E°cell ─
Or, Ecell = E°cell ─
n 96500
n
Cu 2+
Cu 2+
Zn 2+
0.0592
log
Since 2 electrons are transferred in Daniel cell, n=2, Ecell = E°cell ─
2
Cu 2+
Similarly for the cell: Ni(s)/Ni2+(aq)|Ag+(aq)/Ag(s) [whose cell reaction is: Ni(s)+2Ag+(aq) → Ni2+(aq)+Ag(s)]
Ni 2+
0.0592
E.M.F,
Ecell = E°cell ─
log
2
2
Ag +
*** Solve related mathematical problems from mathematical problem section-(B)
Electrodes and Their Classification
The metallic and nonmetallic electric conductors (called electronic conductors) which connect the electronic
conductor and solution (which convey ions) of electrochemical cell are called electrodes. Two electrodes are
needed to construct an electrochemical cell. One is anode and another is cathode.
Classification of Electrodes: Based on construction electrodes are of five types:
(1) Metal and Metal ion electrode
(2) Metal and insoluble metal salt electrode
(3) Metal-amalgam and metal ion electrode
(4) Redox electrode
(5) Gas electrode
(1) Metal and Metal ion electrode: The electrode which is made by dipping a metallic rod into the solution of
that metal is called metal/metal ion electrode. This half-cell is denoted by M(s)/Mn+(aq). Example, Zn(s)/Zn2+
(aq), Cu(s)/Cu2+ (aq), Ag|Ag+(aq) etc.
(2) Metal and insoluble metal salt electrode: In this type, metal is kept in its insoluble salt and another soluble
salt to common negative ion. For example, Ag wire is immerged into AgCl(s) kept in HC1 or NaCl solution to
make half-cell, Ag(s), AgCl(s)/Cl─(aq). Another example is the Calomel electrode: Hg(l), Hg2Cl2(s)/ Cl─(aq).
As anode, calomel half-cell reaction
: 2Hg(l) + 2Cl─
Hg2Cl2 (s) + 2e─
As cathode, calomel half-cell reaction
: Hg2Cl2 + 2e─ 2Hg(l) + 2Cl─(aq)
(3) Metal-amalgam and metal ion electrode: In this type, metal-amalgam rod prepared from reactive metal and
mercury is kept immersed is solution of reactive metal salt. Oxidation of reactive metal is controlled by using its
amalgam like sodium amalgam electrode: Na.Hg (s)/Na+(aq)
Here half-cell reaction:
Na.Hg(s)
Na+(aq)+e─ +Hg(l)
(4) Redox electrode: In this type, one noble metal like Pt or Au wire as metal conductor is dipped is solution of
two salts of a transition metal with two different oxidation numbers. For example, Pt, Fe2+(aq)/Fe3+ (aq)
Here, half-cell reaction is:
Fe2+(aq)
Fe3+(aq)+e─
(6) Gas electrode: In this type, one noble metal like Pt-wire as metal conductor is dipped in solution of
compound of H2 or Cl2 and corresponding gas is passed with 1atm pressure into above mentioned solutions as
bubbles. For example, H-electrode: Pt, H2(g) (1atm)/H+(aq)(1M).
Here, half-cell reaction:
H2(g)
2H+(aq) + 2e─
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
10
Electrochemical Cell
The cell/device which is capable of either generating electrical energy from chemical reactions or facilitating
chemical reactions through the introduction of electrical energy is called electrochemical cell. For example
Daniel cell, dry cell, lead storage battery, Nelson cell (used for manufacturing NaOH), cells used in the
extraction/purification of metals etc.
Classification of Electrochemical cell: Electrochemical cells are of two types: (1) Double chamber
electrochemical cell or Galvanic cell or Voltaic cell; (2) Single chamber electrochemical cell or Electrolytic cell
(1) Double chamber electrochemical cell Galvanic cell or Voltaic cell: The cell in which chemical energy is
converted into electrical energy is called galvanic cell. Example: Daniel cell, dry cell, lead storage battery etc.
(2) Single chamber electrochemical cell or Electrolytic cell: The cell in which electrical energy is converted
into chemical energy is called electrolytic cell. In other words, the cell where electrolysis is done is called
electrolytic cell. Nelson cell (used for manufac-turing NaOH), cells used in the extraction/purification of metals
etc are the examples of electrolytic cell.
Table: Differences between Galvanic cell and Electrolytic cell
Subject
Galvanic cell
Electrolytic cell
1. Definition
Do
Do
2. Energy
Galvanic cell is an electrical energy Electrolytic cell is a electrical energy utilizing
conversion
producing cell.
cell.
3. External
External circuit does not contain any battery Electrolytic cell contains battery with external
circuit
for electricity.
circuit for electrical energy.
4. Salt bridge Salt bridge is needed
Salt bridge is not needed
5. Nature of
In galvanic cell anode is negative and In electrolytic cell anode is positive and cathode
electrodes
cathode is positive.
is negative.
6. Electrode
Two electrodes are in different electrolytes in Two electrodes are in same electrolyte in one
position
different vessels.
vessel.
7. Redox
In galvanic cell redox reaction is In electrolytic cell redox reaction is dependent
reaction
spontaneous and independent.
on external electrical source.
8. Metallic
Different metallic rod is used as anode and Same or different metallic rods can be used as
rod used as
cathode.
anode or cathode.
electrode
9. Function of Electrode (metallic rod) works as electron Electrode (metallic rod) only works as electron
electrode
conveyer and also takes part in reaction.
conveyer and do not take part in any reaction.
Anode and cathode is determined by the Anode and cathode is determined based on the
10.
Determinareactivity of metal. [The more reactive metal connection of the electrode with the electrical
tion of anode acts as anode and the less reactive metal acts source (battery). [The metallic rod, connected to
and cathode
as cathode]
the positive end of the battery, works as anode
and the rod, connected to the negative end of the
battery, works as cathode.]
11. Example
Daniel cell, dry cell, lead storage battery etc. Nelson cell (used to manufacture NaOH), cells
used in the extraction/purification of metals etc.
12. Figure of
cell
Daniel Cell
Anode reaction:Zn(s)→Zn2+(aq)+2e─[Ox]
Cathode reaction:Cu2+(aq)+2e─→Cu(s)
Zn(s) + Cu2+(aq) →Zn2+(aq) + Cu(s)
To generate electricity or electrical energy.
Electrolysis of Molten NaCl
2Na+(l) + 2Cl─(l)
2NaCl(l)
─
Anode reaction: 2Cl (l)→Cl2(g)+2e─[Ox]
Cathode reaction:2Na+(l) + 2e─ → 2Na(s) [Red]
Electroplating in metal, Extraction of reactive
14. Uses
metal, Purification of metal, Production of new
chemical compound.
Construction and Mechanism of Daniel cell (Galvanic cell)
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
11
13. Reactions
In Daniel cell Cu/Cu2+(aq) electrode is used as cathode and Zn/Zn2+(aq) electrode is used as anode. Copper
rod is dipped in CuSO4 solution as cathode in a container and in another container zinc rod is dipped in ZnSO4
solution as anode. A ‘U’ shaped tube full of inert electrolyte (like KCl, KNO3, NH4NO3) solution is dipped into
the two solutions to make the solutions of both the containers connect. Now, if the two electrodes are connected
with wire the following oxidation-reduction reaction will occur spontaneously.
Anode reaction:Zn(s)→Zn2+(aq)+2e─[Oxidation]
Cathode reaction:Cu2+(aq)+2e─→Cu(s) [Reduction]
[Fig: Galvanic cell (See from the above table)]
2+
2+
Zn(s) + Cu (aq) →Zn (aq) + Cu(s)
That means in anode Zn is oxidized to Zn2+ by donating electron and dissolves in solution and in cathode Cu2+
ion of solution settles on the cathode as metallic Cu, accepting electrons from cathode.
In fact the produced electrons of anode reach to cathode by wire and make electronic equilibrium. Thus, if
two electrodes are connected with wire an electron flow will be created from anode to cathode. Electronic flow
means current flow. Therefore, if an electric bulb is connected with the wire of Daniel cell then the bulb will be
lit (enlightened).
Salt Bridge
Q. What is Salt Bridge?
Ans: A salt bridge is an inverted U-shaped glass tube filled with an inert electrolyte (like KCl or KNO3 or
NH4NO3) solution, which connects electrically two electrolytes of a cell indirectly. The inverted U-tube is filled
with concentrated solution of an inert electrolyte like KCl or KNO3 or NH4NO3 and its two ends are packed with
porous cotton or glass wool.
Q. What is inert electrolyte or inactive electrolyte or supporting electrolyte?
Ans: According to IUPAC, inert electrolyte is an electrolyte containing chemical species that are not
electroactive (within the range of potentials used) and which has an ionic strength and conductivity much larger
than those due to the electroactive species added to the electrolyte. For example, KCl, KNO3, NH4NO3 etc.
Q. Explain the activity and necessity of salt bridge in Daniel cell (or, Galvanic cell).
Ans: In the anode of Daniel cell (or, Galvanic cell) Zn is oxidized to Zn2+ by donating electron and dissolves in
solution and in cathode Cu2+ ion of solution settles on the cathode as metallic Cu, accepting electrons from
cathode.
Anode reaction:Zn(s)→Zn2+(aq)+2e─
[Oxidation]
2+
─
Cathode reaction:Cu (aq)+2e →Cu(s)
[Reduction]
Overall:
Zn(s) + Cu2+(aq) →Zn2+(aq) + Cu(s)
[Redox]
2+
Thus in anode container there is abundance of Zn and in cathode container there is deficiency of Cu2+ ion.
We know that, any ion (negative or positive) can not exist freely i.e. a positive ion can not be produced
without the presence of a negative ion and vice-versa. So, equal amount of anion (sulphate ion) will be required
for the Zn2+ ion produced in anode. On the other hand as a result of settling of Cu2+ ion as Cu on the cathode
equivalent amount of negative ion (sulphate ion) will be free in solution. In fact, if the equilibrium does not
exist between the ions of two containers, the reaction will not occur. So, if salt bridge is added salt bridge
containing positive (K+) and negative (Cl─) ion will maintain the imbalance of positive and negative ions in
anode and cathode container. Thus salt bridge maintains an ionic equilibrium in between the anode and
cathode electrolytes.
Functions of salt bridge:
(1) Salt bridge connects the electrolyte solutions of two half cells and completes the cell circuit.
(2) The electrolyte (KC1) of salt bridge does not react with solutions of two half cells. It maintains electrical
neutrality between the two cell liquids.
(3) In Daniel cell with the increase of Zn2+ ions in oxidation half cell, negative ions (Cl─ions) from salt bridge
diffuse into it and similarly with the decrease of Cu2+ ions in reduction half cell, positive ions (K+ ions) from the
salt bridge diffuse into it. Thus electrical neutrality maintains in both half cells. Otherwise cell reaction will be
stopped.
(4) A salt bridge helps to maintain the total charge balance in two half cells.
(5) A salt bridge minimizes or eliminates the liquid junction potential. The unequal migration of positive and
negative ions across the liquid-liquid junction creates a potential difference across the junction, which is called
liquid junction potential.
Construction and Mechanism of Electrolytic cell: Already discussed.
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
12
Rechargeable Battery
The battery/cell which is capable of being charged repeatedly is called rechargeable battery or storage battery or
secondary battery or accumulator. Two important examples of rechargeable battery are (1) Lead storage battery
(2) Lithium storage battery or Lithium ion battery
(1) Construction and Mechanism of Lead storage battery:
The battery used in automobiles like bus, car, trucks etc. is the lead storage battery. This battery consists of a
number of voltaic cells connected in series. Three to six such cells are generally combined to get 6 to 12 volt
battery. In each cell, the anode is a grid of lead packed with finely divided spongy lead and the cathode is a grid
of lead packed with PbO2. The electrolyte is aqueous solution of sulphuric acid (38% by mass) having a density
1.30g.ml─1 sulphuric acid. Lead storage battery (or any storage battery) acts as voltaic cell (Galvanic cell) as well
as electrolytic cell. During discharging it acts as voltaic cell and during charging/recharging it acts as electrolytic
cell. For example, when it is used to start the engine of the automobile, it acts as a voltaic cell and produces
electric energy. During recharging, it acts as an electrolytic cell.
Discharging the battery (i.e. conversion of chemical energy into electrical energy):
When the lead plates are kept for sometimes, lead sulphate is formed on them. At the anode, lead is oxidized to
Pb2+ ions and insoluble PbSO4 is formed. At the cathode, PbO2 is reduced to Pb2+ ions and PbSO4 is formed. The
following reactions take place in the lead storage cell:
At Anode: The lead loses two electrons and is oxidized to Pb2+ ions
Pb(s)→ Pb2+ (aq) + 2e─
Pb2+(aq) + SO42─(aq)→ PbSO4(s)
Overall anode reaction: Pb(s) + SO42─(aq)→ PbSO4(s) + 2e─
At Cathode: The PbO2 is reduced as:
PbO2(s) + 4H+ + 2e─→ Pb2+(aq) + 2H2O
Pb2+(aq) + SO42─(aq)→ PbSO4(s)
Overall cathode reaction:
PbO2(s) +4H++ SO42─(aq) + 2e─→ PbSO4(s) + 2H2O
Thus the complete electrode reactions and overall cell reaction are:
Anode reaction:
Pb(s) + SO42─(aq)→ PbSO4(s) + 2e─
E°=0.3V
Cathode reaction:
PbO2(s) +4H++ SO42─(aq) + 2e─→ PbSO4(s) + 2H2O
E°=1.7V
Overall reaction:
Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O
E°=2.0V
The cell may be represented as:
Pb(s), PbSO4(s)/H2SO4 (aq)/PbO2(s),Pb(s);
E°=2.0V
Anode
Cathode
It is clear from the above reaction that during-the working of the cell, PbSO4 is formed at each electrode and
sulphuric acid is used up. As a result, the concentration of H2SO4 decreases and the density of the solution
also decreases. When the density of H2SO4 falls below 1.2 gmL─1, the battery needs
recharging.
Recharging/ Charging the Battery (i.e. conversion of electrical energy into
chemical energy):
The cell can be charged by passing electric current of a suitable voltage in the
opposite direction. The electrode reaction gets reversed. As a result, the flow of
electrons gets reversed and lead deposited on anode and PbO2 on the cathode. The
density of sulphuric acid also increases. The reaction may be written as:
Cathode (Negative electrode connected with the source): PbSO4(s) + 2e─ →Pb(s) + SO42─(aq)
Anode (Positive electrode connected with the source):
PbSO4(s) + 2H2O→ PbO2(s) + SO42─(aq)+ 4H++2e─
Overall reaction:
2PbSO4(s) + 2H2O→Pb(s) + PbO2(s) + 4H+ + 2SO42─(aq)
Electromotive force of this cell is 2 volt. By applying several cells serially a cell whose e.m.f. is greater can be
made. For example, in the battery used in car 6 cells of this type are arranged serially to make a battery of 12volt.
When the car moves then the cell is charged by the generator. Electricity of cell is used to start a car. If the
engine is switched on and off frequently without driving a car then power of battery decreases. Then the battery
is charged again by applying electrical energy from outside source. During usage of electricity H 2SO4 is used.
Therefore, density of liquid solution decreases. Sometimes condition of the battery can be checked by measuring
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
13
density of a liquid solution by normal hydrometer. Specific gravity of H2SO4 has to be 1.2. On the other hand,
when battery is charged then water which is mixed with H2SO4 is electrolyzed.
Electrolysis
H2(g) + O2 (g)
H2O(l)
Due to evolve of H2(g) and O2(g) amount of water in battery keeps decreasing. Therefore, by adding pure water
to battery density of H2SO4 solution is kept constant to 1.2.
Advantages of Lead Storage Battery: (1) Low internal resistance (2) Rechargeable (3) Checking charge level
(4) Availability (5) If more power is needed, several batteries can be used together (6) it is cheaper and tolerable
to overcharging (7) It remains for a long period of time even preserved without electrolyte (8) It is available in
various size and capacity (9) Can be recycled; about 78% of lead storage battery can be recycled.
Disadvantages of Lead Storage Battery: (1) Dangers from acid burn (2) The electrolyte can undergo
evaporation during charging. H2 gas evolves which is flammable and can cause dangerous situation. (3)
Electrolyte levels (4) Troubles to carry heavy battery (5) Environmental pollution (6) The electrolyte and lead
electrode can cause environmental pollution (7) During discharging lead storage battery cannot be preserved (8)
Not suitable for rapid charging; Characteristic life cycle: 300-500 cycles. (9) The lead used in lead storage
battery is very toxic and carcinogenic (10) Water needs to be added frequently.
(2) Lithium storage battery or Lithium ion battery or Li-ion battery (LIB):
[Note:The battery in which lithium is used can be classified into two classes namely: (i) Lithium battery: It is a
primary battery i.e. not rechargeable. This battery is used in watch, calculator etc. (ii) Lithium ion battery: It is
a secondary battery i.e. rechargeable. This battery is used in cell phone, laptop, digital camera, power tools etc.
Since the lithium battery is a primary battery i.e. not rechargeable, it is not included in syllabus; only lithium ion
battery is included in syllabus and hence is discussed below.]
Construction of Li-ion battery:
1. Anode: Graphite anode with Li atoms inserted between its layers of carbon atoms. This so called lithiated
graphite is written as LixC6.
2. Cathode: CoO2 or MnO2 which incorporate Li+ ions into its structure to form LiCoO2 or LiMn2O4.
3. Electrolyte: The electrolyte is a non aqueous solution of lithium salt (like 1M LiPF6) dissolved in an organic
solvent (such as a mixture of dimethyl carbonate and methyl ethyl carbonate) that can transport Li+ ions.
Mechanism of Li-ion battery:
Discharging process: More reactive Li atoms give up electrons and converted into Li+ ion. Then free electrons
flow through the circuit, while solvated Li+ ions flow from anode to cathode within the cell with electrolyte.
Anode reaction:
LixC6(s) → xLi+(soln) + 6C + xe─
Cathode reaction: Li1─xCoO2(s) + xLi+(soln) + xe─ → LiCoO2(s)
Overall cell reaction:LixC6(s)+Li1─xCoO2(s)→LiCoO2 (s)+6C(s);
Ecell=3.6~3.7V
Charge Process: During recharging, the cell reaction is reversed i.e.
Cathode reaction:
xLi+(soln) + 6C + xe─→ LixC6(s)
Anode reaction:
LiCoO2(s) → Li1─xCoO2(s) + xLi+(soln) + xe─
Overall cell reaction: LiCoO2(s) + 6C(s) →LixC6(s) + Li1─xCoO2(s)
The discharging & charging processes of LIB can be shown simultaneously as
follows:
LixC6(s)+Li1─xCoO2(s)
Discharging
Charging
LiCoO2(s)+ 6C(s)
Q. Why is non aqueous electrolyte used in Li-ion battery?
Ans: Lithium is very reactive. It strongly reacts with water to produce lithium hydroxide and hydrogen gas.
Li + H2O →LiOH + H2
For this reason non aqueous electrolyte is used in Li-ion battery.
Advantages of Lithium Ion Battery: (1) Portable (2) Rechargeable (3) Checking charge level (4) Lighter
weight than lead storage battery (5) High energy density (6) Sealed cell; no need for maintenance (7) Long life
cycle (8) Can function at a wide range of temperature (9) Rapid charge capability (10) High working efficiency
(11) No memory effect (12) Widely used in laptop, cell phone, i-pad etc.
Disadvantages of Lithium Ion Battery: The main drawbacks of lithium-ion battery are (1) expensive (2) flameability of the organic solvent (3) less powerful than lithium battery (4) lithium can lead to explosion (5) More
sensitive at higher temperature (6) Gets destroyed due to full-discharging (7) Ion transport is disrupted due to
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
14
electrolyte deposition during charging, cell efficiency decreases (8) Lower power density is observed in
cylindrical batteries (9) Higher internal resistance.
Fuel Cell
In 1839 British scientist Sir William Robert Grove invented the fuel cell first. Later in 1960 Professor Bacon and
Professor Frost of Cambridge University made the fuel cell commercially. A fuel cell can be defined as the cell
which generates electricity by converting the chemical energy of a fuel and an oxidant into electric energy. In
other words, fuel cell is one type of Galvanic cell in which the chemical energy of a fuel (H 2, CH3OH, CH4) is
converted to electrical energy directly. The main difference between fuel cell (also known as flow battery) and
ordinary battery is that the reactants are not contained within the cell rather these are continuously supplied from
an external reservoir.
Classification of fuel cell: Fuel cells can be classified in different ways:
(A) Based on the variation of electrolyte fuel cells are of six types:
1. Proton Exchange Membrane Fuel Cell (PEMFC)
2. Phsphoric Acid Fuel Cell (PAFC)
3. Solid Oxide Fuel Cell (SOFC)
4. Alkaline Fuel Cell (AFC)
5. Molten Carbonate Fuel Cell (MCFC)
6. Direct Methanol Fuel Cell (DMFC)
(B) Based on the fuel used fuel cells are of four types:
7. Hydrogen-Oxygen fuel cell
8. Hydrazine-Oxygen fuel cell
9. Hydrocarbon-Oxygen fuel cell
10. Methanol-Oxygen fuel cell
(C) Based on the temperature range in which fuel cells are operated, fuel cells are of four types:
1. Low temperature fuel cell (25-100°C) e.g. Hydrogen-Oxygen fuel cell
2. Medium temperature fuel cell(100-500°C) e.g. Hydrogen-Oxygen fuel cell, Natural gas-Oxygen fuel cell
3. High temperature fuel cell (500 - 1000°C) e.g. Alcohol (methanol)-Oxygen fuel cell
4. Very high temperature fuel cell (1000°C and above) e.g. Butane-Oxygen fuel cell
(D) Based on the physical states of fuel used, fuel cells are of three types:
1. Gaseous fuel cell (hydrogen, lower hydrocarbon)
2. Liquid fuel cell (alcohols, hydrazine, higher hydrocarbons)
3. Solid fuel cell (metals)
Hydrogen fuel cell or Hydrogen-Oxygen fuel cell
The hydrogen-oxygen fuel cell was first used as a source of electric power in space vehicles Appolo. This cell
contains porous carbon electrodes (anode and cathode) in which nano-particles of a
Pt-based catalyst remains deposited. The fuel H2 and oxidant O2 do not react directly.
They are flown into separate cell compartments. Both the electrodes remain dipped in
the electrolyte, hot aqueous KOH.
In contact with electrolyte KOH, the fuel H2 donates electrons and is oxidized at anode
and electrons flow in the outer circuit. At the cathode O2 receives two electrons and is
reduced in presence of H2O. The overall cell reaction is simply the conversion of
hydrogen and oxygen to water.
Anode reaction: 2H2 (g) + 4OH─(aq) → 4H2O(l) + 4e─
E° = + 0.83V
Cathode reaction: O2 (g) + 2H2O (l) + 4e─→ 4OH─(aq) E° = + 0.40V
Overall cell reaction: 2H2(g) + O2(g) → 2H2O (l)
E° = + 1.23V
In modern fuel cells, the aqueous KOH electrolyte is replaced by a special
polymer membrane that conducts proton but not electrons (Fig). So this type of
fuel cell is called proton exchange membrane fuel cell or PEMFC. The
reactions involved in PEM fuel cell are as follows:
Anode reaction
: 2H2(g) → 4H+(aq)+4e─
Cathode reaction
: O2 (g) + 4H+(aq) + 4e─ → 2H2O (l)
Overall cell reaction : 2H2(g) + O2(g) → 2H2O (l) E° = 1.23V
In PEM cell, both the porous graphite electrodes are impregnated with nanoparticles of Pt-catalyst. Both the electrodes are embedded (fixed) in a polymer
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
15
electrolyte membrane. The polymer has tetrafluoro ethylene backbone ([—F2C—CF2—]n). This chain or
backbone contains sulfonic acid groups (R-SO3─) which play a key role in carrying protons from anode to
cathode.
Mechanism of Reactions in PEMFC: At the anode, two H2 molecules absorb onto the catalyst Pt-metal and are
split and oxidized to four H+ ions and four electrons (e─). These electrons travel through the external wire to the
cathode, while H+ ions become hydrated and migrate through the electrolyte as H3O+ ions to cathode. Then at the
cathode an O2 molecule absorbed onto catalyst Pt-metal accepts an electron (e─) to form O2─ ion which gains one
proton (H+) from H3O+ ion to form HO2 (that is H-O-O). After that it accepts second electron and a proton to
form first H2O molecule and an oxygen atom [O]. That oxygen atom, in similar way, first forms OH ─ ion and
then second H2O molecule. Water molecules thus produced leave the cell.
Important features of some important fuel cells:
Fuel Cell
Electrolyte, Anode,
Fuel used
Half Cell Reactions
Cathode and
Temperature
Proton
Polymer membrane
Reductant: H2
Anode:2H2→ 4H++4e─
Exchange
PEM,
Oxidant: O2
Cathode:O2(g)+4H+(aq)+4e─→2H2O(l)
Membrane
Anode: Graphite Li
Fuel Cell
atoms inserted(LixC6)
(PEMFC)
Cathode: LiCoO2 or
LiMn2O4
Temp: 60°C
Direct
Polymer membrane
Reductant : CH3OH
Anode: 2CH3OH+2H2O→2CO2+12H+
Methanol Fuel PEM
Oxidant: O2
+ 12e─
Cell (DMFC)
Anode: Pt
Cathode : 3O2 +12H+ +12e─→6H2O
Cathode:Pt
Temp: 60°C
Alkaline Fuel
KOH solution,
Reductant : H2
Anode: 2H2+ 4OH─ → 4H2O + 4e─
Cell (AFC)
Anode: Pt
Oxidant : O2
Cathode: O2+4H2O + 4e─ →4OH─
Cathode:Pt
Temp: 150°C
Phsphoric
H3PO4 acid
Reductant : H2
Anode: 2H2(g) →4H+ + 4e─
Acid Fuel Cell Anode: Pt
Oxidant : O2
Cathode: O2(g) + 4H+ + 4e─→2H2O(g)
(PAFC)
Cathode: Pt
Temp: 180°C
Molten
Lithium potassium
Reductant : H2
Anode: 2H2 + 2CO32─→2H2O +
Carbonate Fuel carbonate,
Oxidant : O2
2CO2+4e─
Cell (MCFC)
Anode: Ni
Cathode: O2 + 2CO2 + 4e─→2CO32─
Cathode: Ni
Temp: 650°C
Solid Oxide
Zirconium Oxide (ZrO2) Reductant : H2
Anode: H2 + O2─→ H2O+2e─
Fuel Cell
at 800-1000°C
Oxidant : O2
Cathode: ½O2+4H++2e─→ O2─
(SOFC)
Anode: Pt
Cathode: Pt
Advantages and Disadvantages of Hydrogen Fuel Cell:
Advantages:
1. Hydrogen fuel cells can convert about 75% of the fuel's bond energy into useable electric power in contrast
to 40% for a coal-fired-power plant and 25% for a gasoline-powered car engine.
2. Fuel cells can be installed near the use point, thus reducing electrical transmission requirements and
accompanying losses.
3. They have few mechanical components; hence they operate fairly quietly and require little attention and
maintenance.
4. In fuel cell the only emission is harmless water and hence its pollutant emission is zero.
5. There is no requirement for large volumes of cooling water such as are necessary to condense exhaust gas
system from a turbine in conventional power plant.
6. As fuel cells do not make noise, they can be readily accepted in residential areas.
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
16
7. It is a renewable source of energy.
8. Hydrogen fuel cells have been used for years to provide electricity and pure water during space flight.
9. In near future, hydrogen fuel cells will supply electric power for transportation, hospitals, hotels, residential
apartment buildings and commercial needs. Of course, their overall environmental impact will depend on
how the H2 gas is obtained. For example, water electrolyzing with solar power will have a negligible impact.
But water electrolyzing with electricity from coal-fired plant will have much pollution.
Disadvantages:
1. High initial cost and low service life.
2. The fuel cell uses oxygen and hydrogen gas to produce electricity. So it requires a huge storage facility.
3. The hydrogen is not so readily available. However hydrogen has some limitations that make it impractical for
use in most applications.
4. Hydrogen and oxygen is difficult to store and distribute, so it would be much more convenient if fuel cells
could use fuels that are more readily available.
Measurement of pH of a Solution by pH meter: [Details: See any Chemisty 2nd paper book]
Mathematical Problems
(A) Faraday’s Law Related Problems:
1. How many coulombs of charge will be needed for the following reduction reactions: (i) 1mol Na + →Na
[Ans: 96500C]; (ii) 0.5mol Al3+→Al [Ans: 144750C]; (iii) 0.25mol MnO4─→Mn2+[Ans: 120625C]; (iv)
1mol Cr2O72─ → Cr3+ [Ans: 2.897×105C]
2. How many coulombs of charge will be needed for the following oxidation reactions: (i) 1mol FeO →Fe 2O3
[Ans: 96500C]; (ii) 1mol H2O →O2 [Ans: 1.93×105C]; (iii) 1mol NaClO3 → NaClO4 [Ans: 96500C].
3. How much copper will be deposited, when 5A current is passed through CuSO4 solution for 60 minutes?
[Ans: 5.922g Cu]
4. How much copper will be deposited on cathode, if 0.5 A current is passed through blue vitriol solution for 10
minutes? [Ans: 0.0987 g]
5. How much silver will be deposited at the cathode, if a current of 0.2A is passed through a AgNO 3 solution
for 50 minutes? [Ans. 0.6715g]
6. How much nickel will be deposited at the cathode when a current of 5A strength is passed through Ni((NO3)2
solution for 30 min.? [Ans. 2.737g]
7. 160 mA current is passed through CuSO4 solution for 40 minutes. Calculate amount of copper atoms
deposited at the cathode. [Ans: 1.198159585×1021 Cu atoms]
8. How many Ca atoms will be deposited at he cathode, when 25 mA current is passed through CaCl2 solution
for 60 seconds? [Ans. 4.68×1018 atoms]
9. If 50mA current is passed through a molten NaCl for 2 hrs, how many Na+ ions will be deposited at cathode?
[Ans: 2.24692×1021]
10. If 0.75A current is passed for 1min 20 sec, how many H+, Ni2+ and Al3+ ions will be deposited at cathode?
[Ans: H=3.74×1020, Ni=1.87×1020, Al=1.24667×1020]
11. If a current of 1.5A strength is passed through a CuSO4 solution for 10 minutes, then 0.2964g Cu will be
deposited at cathode. Calculate the atomic mass of Cu. [Ans: 63.56]
12. If a current of 1.5A strength is passed through an electrolytic solution for 54 minutes 58sec, then 1.5g metal
of valency 2 will be deposited at cathode. Calculate the atomic mass of the metal. [Ans: 58.5]
13. If a current of 7.5×102mA strength is passed through an electrolytic solution of divalent metal for 5 minutes,
then the mass of the cathode is increased by 74.0285mg. Calculate the atomic mass of the metal. [Ans: 63.5]
14. If 2A current is passed through a AgNO3 solution for 30 min, 4g Ag is deposited at cathode. Calculate the
electrochemical equivalent of Ag. [Ans: 1.229×10─3g.C─1]
15. If 1.5A current is passed through a CuSO4 solution for 10 min, 0.2964g Cu is deposited at cathode. Calculate
the electrochemical equivalent of Cu. [Ans: 3.2933×10─4g.C─1]
16. 500 mL H2 at STP is produced by electrolysis of aqueous H2SO4 acid with platinum electrodes. How many
coulombs of electricity are needed for that? [Ans: 4308.036 C]
17. 250 mL hydrogen gas at STP was produced by passing electricity through aqueous dilute H2SO4 for one
hour. What was the strength of the current? [Ans: 0.5083 A]
18. How long a current of 1.5 ampere should be passed through a silver nitrate solution to deposit 1.89g silver?
[Ans: 18 min 47.08 sec]
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
17
19. How long should a current of 0.12A be passed through a Cr2(SO4)3 to deposit 1g chromium? [Ans: 12 hr 53
min 14 sec]
20. How long a current of 2 ampere strength should be passed through a copper sulphate solution to deposit
2.368 g copper? [Ans: 60 min.]
21. How long a current of 1.5A strength should be passed through molten AlC13 to deposit 1.6 g Al metal? [Ans:
3.177 hrs]
22. How long a current of 2.5A strength should be passed through a dilute H2SO4 solution to produce 600 mL H2
gas at STP? [Ans: 34.46 min]
23. A current was passed through water acidified with H2SO4 for 1.5 hour; when 500 mL H2 gas at STP was
produced. What was the strength of the current? [Ans: 0.7978A]
24. When a current of 0.0422A is passed for 1 hour through a chromium (III) sulphate solution, 0.0275g
chromium is deposited on the cathode. What is the charge of chromium ion? [Ans: +3 i.e. Cr3+ ion]
25. A current of 0.1A strength is passed through a metal ion solution for 160 minutes to deposit 0.295g metal.
What will be charge of the metal ion? [Atomic mass of the metal = 58.7] [Ans. Ni2+]
26. A current of 3.7A strength is passed through 0.5 L solution of 2M Ni(NO 3)2 solution for 6 hrs. What will be
the strength of the solution after electrolysis? [Ni = 58.7] [Ans: 1.172M]
27. A current of 2.5A strength is passed through 0.5L solution of 1M Ni(NO3)2 solution for 2hrs. What will be
the strength of the solution after electrolysis? [Ni = 58.7] [Ans: 0.8135M]
(B) Electrode and Cell Potentials Related Problems:
28. Explain whether the following reaction will occur spontaneously or not?
(i) Cu(s) + ZnSO4(aq) → CuSO4(aq) + Zn(s)
(ii) Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
(iii) 2Ag(s) + Zn2+(aq)→ 2Ag+(aq) + Zn(s)
(iv) Fe(s) + Zn2+(aq) → Fe2+(aq) + Zn(s)
3+
2+
(v) 3Sn(s) + 2Fe (aq) → 3Sn (aq) + 2Fe(s)
(vi) 2Al(s) + 3ZnSO4 (aq) → Al2(SO4)3(aq) +3Zn(s)
+
2+
2+
[Given, E°Ag /Ag = +0.799V; E°Zn /Zn = ─0.76V;
E°Cu /Cu = +0.34V; E°Fe2+/Fe = ─ 0.44V; E°Fe3+/Fe =
2+
2+
─0.036V;E°Mg /Mg=─2.37V ; E°Sn /Sn = ─0.14V; E°Al3+/Al=─1.662V ]
29. Explain, whether the MgSO4 solution will be stored in copper vessel or not. Given, E°Cu2+/Cu = +0.34V,
E°Mg2+/Mg = ─2.3V.
30. Explain, whether FeSO4 soln will be stored in Zn vessel or not. Given, E°Fe/Fe2+ =+0.44V,E°Zn/Zn2+= +0.76V.
31. Explain, whether FeSO4 solution will be stored in copper vessel or not. Given, E°Cu/Cu2+ =─0.34V and
E°Fe/Fe2+ = + 0.44V.
32. Write the cell diagram of the cell with silver electrode and cadmium electrode and identify the anode and
cathode. Write half cell reactions and total cell reaction and calculate e.m.f of the cell. Given, E° Cd2+/Cd =
─0.40V and E°Ag+/Ag= + 0.80V [Ans: Ecell = +1.2V]
33. In between CuSO4 and ZnSO4, which one can be stored in iron vessel? Given, E°Cu/Cu2+ =0.34V, E°Zn/Zn2+= +0.76V
and E°Fe/Fe2+ = + 0.44V.
34. If E°Zn2+/Zn= ─0.763V, E°Pd2+/Pd= ─0.126V and both the solutions are 1M then whether Zn2+ ion will be
reduced by Pd metal or not-Explain [Ans: Ecell =─0.637V, so not possible]
35. The oxidation potentials of zinc electrode and silver electrode are +0.76V & ─0.799V. Calculate the e.m.f of
the cell. [Ans: 1.559 V]
36. With the help of Nernst equation, calculate the e.m.f of the following cell:
Mg(s)/Mg2+(0.001M) |Cu2+(0.0001M)/Cu(s); Given, E°Mg2+/Mg=─2.37V and E°Cu2+/Cu=+0.34V. [Ans: 2.6804V]
37. At 25°C, calculate the e.m.f of the following cell: Fe(s)/Fe2+(0.001M) || H+(1M)/H2(1 atm), Pt; Given,
E°Fe2+/Fe = ─ 0.44V. [Ans: 0.5288V]
38. At 25°C, calculate the e.m.f of the following cell: Sn(s)/Sn2+(0.05M) || H+(0.02M)/H2(1atm), Pt; Given,
E°Sn2+/Sn = ─0.14V. [Ans. 0.07793V]
39. At 25°C, calculate the e.m.f of the following cell: Pb(s)/Pb2+(lM) || H+(0.4M)/H2(1 atm), Pt; Given, E°Pb /Pb2+
= 0.14V. [Ans. 0. 1165 V]
40. If the concentrations of Zn2+ and Cu2+ ions are 0.1M and 0.05M respectively, calculate the cell potential of
Daniel cell. Given at 25°C, E°Zn2+/Zn = ─0.76V, E°Cu2+/Cu = +0.34V. [Ans:1.0911V]
41. At 25°C, calculate the e.m.f of the following cell: Zn(s)/Zn2+(0.001M) || Ag+(0.1M)/Ag. Given, E°Zn2+/Zn=
─0.763V and E°Ag+/Ag= + 0.80V. [Ans: 1.592V] [Hints: Zn+2Ag+ →Zn2+ + 2Ag; n=2, ln{[Zn2+]/[Ag+] 2}]
42. At 25°C, calculate the e.m.f of the following cell: Cr(s)/Cr3+(0.5M) || Fe2+(0.8M)/Fe. Given, E°Fe2+/Fe=
─0.44V and E°Cr3+/Cr= ─ 0.71V. [Ans: 0.277V] [Hints:2Cr+3Fe2+ →2Cr3++3Fe; n=6, ln{[Cr3+] 2/[Fe2+] 3}]
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
18
43. At 25°C temperature, if a Zn electrode is dipped into a 0.01M solution of Zn2+ ion, then what will be the
electrode potential? , Given, E°Zn/Zn2+= +0.76V. [Ans: 0.8221V] [Hints: Zn →Zn2+ + 2e─; EZn/Zn2+ = E°Zn/Zn2+
Zn 2+ (aq)
2.303RT
log
─
; Here, [Zn(s)]=1, [Zn2+(aq)]=0.01M ]
nF
Zn (s)
44. At 25°C temperature, if a Cu electrode is dipped into a 0.15M solution of Cu 2+ ion, then what will be the
electrode potential? Given, E°Cu/Cu2+= ─0.34V [Hints: Cu →Cu2+ + 2e─]
45. At 25°C temperature, if a Ag electrode is dipped into a 0.2M solution of Ag2+ ion, then what will be the
electrode potential? Given, E°Ag/Ag+= ─0.799V; [Ans: ─0.840344V][Hints: Ag →Ag+ + e─]
46. A zinc rod is dipped into 0.1M ZnSO4 solution at 298K temperature and hence 95% of salt dissociate.
Determine the electrode potential [Given, E°Zn2+/Zn = ─0.76V]. [Ans: ─0.79V V] [Hints: Zn →Zn2+ + 2e─;
Zn 2+ (aq)
2.303RT
2+
2+
log
EZn /Zn = E°Zn /Zn ─
; Here, [Zn(s)]=1, [Zn2+(aq)]=0.1×95/100=0.095M ]
nF
Zn (s)
Board questions [Chapter 04: Electro-chemistry]
Knowledge Based Questions
1. State Faraday’s first law? [Co-15, A-18, D-19, B19]
2. What is Faraday’s constant? [S-17]
3. What is electrochemical equivalent? [C-15]
4. Define e.m.f. [B-15]
5. What is electromotive force or emf?[C-17, R-19,
J-19, S-19]
6. What is standard electrode potential? [R-15, C-19]
7. What is electrode? [D-16]
8. What is reference electrode?[D-17, Di-17]
9. What is primary reference electrode? [R-19]
10. What is called secondary electric cell? [C-19]
11. What is electrochemical cell?[R-17,D-19]
12. What is electrolytic cell? [S-15]
13. What is salt bridge? [B-15, D-16 S-17]
14. What is fuel cell? [J-15, R-17, B-19]
15. What is SOFC? [Di-17]
Understanding Based Question
1. Why is NaCl called electrolytic conductor?[Co15]
2. Why is NaCl(aq) electrolyte?-Explain. [D-19]
3. Why does the current of the electronic
conductance
decrease
with
increasing
temperature? [C-19]
4. "Acid mixed water is an electrolytic conductor”Why? [C-16]
5. Electrochemical
equivalent
of
Ag
is
0.001118gC─1. What does it mean? [B-15,C-16,J16]
6. The reduction potential of Zn is ─0.76V-What
does it mean?[R-16]
7. Oxidation
potential
of
zinc
electrode,
2+
E°Zn/Zn =+0.76V; What does it mean? [Di-17]
8. Galvanic cell gives redox reaction-Explain[Co-16]
9. Why is electrolysis a redox reaction?[D-17, S17,A-18]
10. What do you mean by oxidation half cell? [Co-17]
11. Why Cu doesn’t react with dil. H2SO4?[Co-17]
12. What is standard hydrogen electrode? [B-17]
13. Why is hydrogen electrode called primary
reference electrode? [Co-15, B-19]
14. What do you mean by standard hydrogen
electrode? [C-15]
15. How many chambers in a Galvanic cell? Explain.
[Co-19]
16. Why is Zn act as a reducing agent in Daniel cell?
[S-19]
17. Explain the role of salt bridge. [D-15]
18. Why is salt bridge used in electrochemical cell?
[B-17, Di-19, B-19, S-19]
19. Explain the importance of salt bridge. [J-15]
20. Why is water added to storage battery before
charging? [Di-16]
21. What is the advantage of using lithium ion
battery? [S-15]
22. Why Lithium ion battery is more useful than
Lead-storage battery? [J-19]
23. Why is fuel cell environment friendly?[D-17,R17, Co-19]
24. Corrosion is a chemical process - Explain. [R-19]
Creative Question (CQ)
CQ-1: Standard electrode potential of some
elements and a cell: E° Cu/Cu2+ =─0.34V, E° Fe/Fe2+=
0.44V and E° Zn/Zn2+= 0.76V.
[Di-15]
c. If 250A current is passed through the above cell
for 40 minutes then how many grams of metal will
be deposited at the cathode?
d. In between Zinc and Copper vessels, in which
vessel the solution of the above stem can be
stored?-Analyse
CQ-2: [C-17]
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
19
c. How long it will take to increase 1g mass of
cathode after passing electricity through the
solution of the stem?
d. Verify the possibility to preserve the electrolytic
solution of the stem in a vessel made by metal 'M'
for a long period.
CQ-3: In chemistry laboratory demonstrator
ordered the lab assistant to store a Ni-salt into a
Copper vessel. Unfortunately the lab assistant
stored the Ni-salt into a Zinc vessel. The oxidation
potential of Ni and Zn are +0.25V and
+0.76Vrespectively. [B-15]
c. If 0.1A current is passed for 60 minutes through
the solution of the above mentioned salt then how
much metal will be deposited at the cathode?
d. Can the above electrolyte be stored in zinc vessel
for long time?-Analyse with emf.
CQ-4:
[B-16]
[Atomic mass of M′=108, M′′=63.5 and M′′′=52]
The pH of M′′SO4 solution is less than 7-Explain
d. If 50C charge is passed through the cells 1 and 2,
different amount of substances are deposited at
different electrode-Analyse
CQ-5:
[Co-16]
c.
d. Is it possible to determine the concentration of
H2SO4 solution with the information given in the
stem?-Analyze mathematically.
CQ-7: i. Al(s)/Al3+(aq) || X2+(aq)/X(s) [S-19]
ii. Al(s)/Al3+(aq) || Y2+(aq)/Y(s)
E°X/X2+ =+0.14V, E°Al/Al3+ =+1.66V, E°Y/Y2+ =+0.25V
c. What amount of metal will be decomposed if 0.2A
electricity for 25 minutes passes through the
electrolyte of anode of stem-(i)?
d. Which one of cell (i) and cell (ii) will generate
more electricity?-Analyze mathematically.
CQ-8: [S-17]
c. Determine the EMF of the cell shown in Fig-1.
d. With cell reactions analyse the differences
between Fig-1 and Fig-2
CQ-9:
[Co-15]
[Given, E°Mn/ Mn2+=+1.18V and E°Al/Al3+=+1.66V]
c. Write down the half cell reaction and cell reaction
that take place in the above Al-vessel.
d. ‘The above vessel becomes perforated/decayed
after some days’-Analyse
CQ-10:
[J-17]
c. Write down the cell reaction that take place in the
vessel-A of the stem.
d. Will the above vessel be perforated/decayed after
some days?-Analyse
CQ-11:
[C-15]
c. How much metal will be deposited at cathode if
50A current is passed through the cell of Figure(1) for 10 min?
d. Although both of the Figure-(1) and Figure-(2)
indicate cell but their transformations of energy
are different-Anlyse
CQ-6: The cell potential of the cell given below is +
0.42V.
[B-19]
Pt, H2(g) (1 atm,25°C)/H2SO4(aq)||CuSO4,(aq)/Cu(0.1M)
c. Determine the amount of Cu deposited if 0.2A
electricity is passed for 2 minutes in the cell of the
stem
c. Calculate the standard electromotive force of the
cell of the stem.
d. "Electrolyte solution of the reduction half cell of
the stem should not be kept in a zinc vessel".—
Justify mathematically. [E°zn/zn2+ = 0.76V]
CQ-12:
[B-17]
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
20
c. Mentioning the cell reaction of the above cell
determine the e.m.f of the cell.
d. Is it possible to store the solution of the anode in
zinc vessel?-Analyse mathematically
CQ-13:
[R-15]
c. Find out the total cell potential (emf) of the above
cell.
d. Complete the reactions that take place in the
above cell and present the cell diagram.
CQ-14: Observe the following figure: [S-15]
Calculate the emf of the above cell.
d. ‘The electrolyte of the reduction half cell of the
above mentioned cell should not be kept in zinc
vessel’-Analyse this statement
CQ-15:
[J-16,S-16]
c.
The standard reduction potentials of Nickel, Silver
and Zinc are ─0.25V, +0.799V and ─0.76V
respectively
c. Write down the half cell reaction and cell reaction
taking place in the above mentioned cell.
d. Is it possible to store the solution, used at anode of
the above mentioned cell, in zinc vessel?-Analyse
mathematically
CQ-16: (i) E°A2+(aq)/A(s) = +0.20 volt
[A-18]
(ii) E°B2+(aq)/B(s) = ─0.62 volt
(iii) E°X2+(aq)/X(s) = ─0.80 volt
c. Determine the electromotive force of the cell
consists of number (i) and (ii) half cells.
d. In which vessel made by the 'A' and 'X' metal, the
solution of B2+ ion will be preserved?-Give
mathematical logic.
CQ-17: Oxidation potentials of some metals are:
(i) A2+(aq)/A(s) = +0.40V
[R-17]
3+
(ii) B (aq)/B(s) = +1.66V
(iii) P2+(aq)/P(s) = +0.44V
c. Calculate the total cell potential of the cell formed
by connecting the solutions-(i) and (ii) with the
help of a salt bridge.
d. In between the vessels of A and B in which vessel
the solution-iii can be stored safely?-Analyse with
the order of reactivity.
CQ-18: [R-19]
c. Write down half cell reaction and cell reaction of
the cell in the stem.
d. Will the solution of anode be kept in a container
of zinc for a long time - Analyze mathematically.
CQ-19: A: E°A/A2+= +0.76V; B: E°B/B2+= +0.25V;
C: E°C/C2+= ─0.34V [Co-19]
c. Describe the cell diagram with reaction of the cell
formed by 'A' and 'C'.
d. Is it possible to store solution B2+ into the metallic
vessel of A? Analyze mathematically.
CQ-20:
[Di-19]
c. Determine the e.m.f. of the cell mentioning cell
reaction in the stem.
d. In the stem, whether the solution of anode can be
stored in vessel D-Explain mathematically.
CQ-21: [J-19]
c. Determine the changed concentration of solution
in Pot-A according to stem.
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
21
d. Will there be any problem for using the both cells
for long time?-Analyze mathematically.
CQ-22:
[D-17]
Calculate the electromotive force of the stem.
d. Express your opinion, if there will be any
hindrance to produce electricity for a long time
from the cell.
CQ-23:
[D-15]
c. If the concentrations of Sn2+ and Al3+ are 0.15M
and 0.25M respectively then calculate the
electromotive force of the cell
d. Explain the mechanism of conducting electricity
of the above mentioned cell
CQ-28:
[C-16]
c.
(E°Fe/Fe2+= +0.44V and E°Cu/ Cu2+= +0.34V, T=298K)
c. Calculate the emf of the above cell.
d. If the vessel–B is made of iron then give your
opinion in preserving the above cell for long time.
CQ-24: [C-19]
[Atomic No. of A =12; Atomic No. of B = 24, E°A/A2+
= + 2.36V and E°B/B3+ = + 0.74V]
c. Determine the electromotive force of the cell in
the stem.[Ans: +1.635V]
d. The solution of B3+ ion whether can be stored in
zinc vessel—Explain mathematically. [Ans:
+0.02V, So, cannot be strored]
CQ-25: X/X2+(0.15M)║Y+(0.2M)/Y;
[Di-17]
+
2+
E° Y /Y= +0.80V, E° X /X = ─0.14V;
Temperature=298K
c. Determine the potential of the cell of the stem.
d. In the above mentioned cell how is chemical
energy converted to electrical energy?-Explain
CQ-26:Fe/Fe++(0.13M)║Ag+(0.0004M)/Ag [Co-17]
T=25°C, E° Fe++/Fe = ─0.44V, E° Ag+/Ag= +0.80V
c. Determine the electromotive force of the above
mentioned cell.
d. What difference will be observed in between the
cells which are formed by connecting the half
cells of the stem separately with standard
hydrogen electrode?-Analyse
CQ-27: Al(s), Al3+(aq)║Sn2+(aq),Sn(s)
[R-16]
E° Al3+/Al= ─1.66V and E° Sn2+/Sn= ─0.14V
c. Show the reaction occurred in anode of the stem
cell.
d. Analyse logically the necessity of flow of
electricity to occur reaction in the stem cell.
CQ-29:
[D-16]
c. What would have been the product if CaCl2 ware
used in the above cell?-Explain with cell reaction.
d. Explain the principle of the alkali produced in the
above cell and analyse the reaction occurred in the
above cell
***
CQ-30:
[Co-17]
c. Calculate the amount of substance that is
deposited at the cathode when 5amp current is
passed through cell-B for 10 min.
d. Describe the mechanism of keeping the above cell
active by showing the charging and discharging
reactions of A-cell.
CQ-31:
[S-17]
c. Explain the charging and discharging cell
reactions of Fig-B.
d. Analyse the advantages and disadvantages of the
Fig-A and Fig-B.
CQ-32: ‘A’ and ‘B’ are two rechargeable batteries.
The battery ‘A’ is used in IPS, motor vehicles etc.
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
22
On the other hand the battery ‘B’ is used in laptop,
cell phone etc.
[J-15]
c. Write down the cell reactions of A.
d. ‘A’ is more environment friendly compared to
‘B’?-Analyse
CQ-33: Components of cell-1:Pb, PbO2, H2SO4
Components of cell-2: Li, LiCoO2,Ethylene carbonate
Components of cell-3: H2, O2, KOH
[D-19]
c. Describe the charging and discharging process of
cell-2.
d. Which cell is more environment friendly formed
by the components of cell-1 and cell-3? Analyze.
CQ-34:
[Di-17]
c. Write down the anode, cathode and overall cell
reactions for the above mentioned cell.
d. The above cell is environment friendly but will it
be economic?-Give logic in favor of your answer
Δ
A(g) + KCl(s)
CQ-35: (i) Potassium chlorate
(ii) Zn(s) + H2SO4(dil.) → B(g) + ZnSO4 [J-17]
c. How many grams of reactant will be needed to
prepare 0.07g A of the stem?[Ch-03]
d. What will be the nature of the cell formed by A
and B gases of the stem?-Analyse [Hints:
Hydrogen fuel cell]
Multiple Choice Questions (MCQ)
Dhaka Board-2015
■ Look at the figure below & answer the following
two questions:
5. What is the e.m.f of the above mentioned cell?
a) 0.76V
b) 1.10V
c) 1.23V d) 2.03V
6. Which one is the correct cell reaction or the above
cell?
a) 2H2 + O2 →2H2O
b) 2H2O→2H2 + O2
c) H2 + O2 →H2O2
d) H2O +1/2 O2→H2O2
9. Which one is least reactive metal?
a) Gold
b) Platinum c) Silver d) Chromium
14. How much chromium will be deposited at the
cathode, if 3F charges are passed through a chromium
sulphate solution? [Atomic mass of Cr=52]
a) 17.33g b) 52g
c) 104g
d) 156g
34. Which one of the following cells is environment
friendly?
a) Fuel cell
b) Lead storage battery
c) Cadmium battery d) Lithium ion battery
35. Which one of the followings is primary reference
electrode?
a) Standard hydrogen electrode
b) Calomel electrode c) Glass electrode
d) Cadmium electrode
Chittagong Board-2015
9. What is the cathode reaction of the cell:
Zn | ZnSO4(1.0M) || CuSO4(1.0M) | Cu?
a) Cu ─ 2e─ = Cu2+
b) Zn─2e─ = Zn2+
2+
─
c) Cu + 2e = Cu
d) Zn2+ + 2e─ = Zn
11. Electromotive force of a cell depends on —
i. concentration of ions ii. pressure of gas
iii. reduction and oxidation potential
Which one is correct?
a) i & ii
b) i & iii
c) ii & iii d) i, ii & iii
33. What is the specific gravity of H2SO4 in leadstorage battery?
a) 1.25
b) 1.20
c) 1.15
d) 1.10
34. What is the value of the potential of standard
Hydrogen electrode?
a) +1.00V b) ─0.34V c) 0.00V d) +0.76V
35. In which of the following fuel cell, liquid
electrolyte is absent? a) PEMFC b) AFC
c)
PAFC d) MCFC
Rajshahi Board-2015
1. How much copper will be deposited at the cathode,
if 5A current are passed through a CuSO4 solution?
a) 9.87g
b) 4.96g c) 0.985g d) 0.496g
10. Which one is used as primary reference electrode?
a) Standard hydrogen electrode
b) Calomel electrode
c) Glass electrode d) Cadmium electrode
■ Look at the stem below and answer the following
two questions: Three electrodes and their electrode
potentials are listed below:
Zn(s)/Zn2+(aq)
E°=+0.76V
Fe(s)/Fe2+(aq)
E°=+0.44V
2+
Cu(s)/Cu (aq)
E°=─0.34V
Two cells consisting of the above three electrodes are:
Zn(s)/Zn2+(aq)|| Fe2+(aq)/Fe(s)
Zn(s)/Zn2+(aq)|| Cu2+(aq)/Cu(s)
16. Positive ion will enter the solution from which
electrode in the above two cells??
a) Cu(s)/Cu2+(aq) & Fe(s)/Fe2+(aq) b) Cu(s)/Cu2+(aq)
c) Fe(s)/Fe2+(aq)
d) Zn(s)/Zn2+(aq)
17. The cell potentials in the above two cells are
respectively-a) +0.20V, +0.42V b) +0.32V, +0.42V
c) +0.32V, +1.10V
d) ─0.32V, ─1.10V
24. Sometimes water is added to lead storage battery.
The reason is -a) to keep the cell cooled
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
23
b) to maintain proper pH
c) to maintain the efficiency of battery
d) to maintain the specific gravity of H2SO4
25. The standard reduction potentials of M, N, P and
Q are ─2.92V, ─1.66V, + 0.80V and +1.36V
respectively. Which one is more reactive?
a) M
b) N
c) P
d) Q
Jessore Board-2015:
2. In ohm unit what is the internal resistance of lead
storage battery?
a) 0.80
b) 0.50
c) 0.02
d) 0.001
13. In case of electrolysis which one is correct?
a) Nonmetal deposits at anode & metal at cathode
b) Metal deposits at anode & nonmetal at cathode
c) Electron flows from cathode to anode
d) Concentration of solution remains unchanged
19. Which one of the followings does occur at anode?
a) Deposition of metal ion
b) Discharging of metal ion
c) Reduction
d) Oxidation
■Read the stem and answer the next two questions:—
23. Which one of the followings is used in salt
bridge?
a) CaCl2
b) CuCl2 c) KCl d) Al2(SO4)3
35. The emf of the cell which is constructed by the
half cells: EoM2+ /M =+0.34V and EoM2+ /M = ─2.30V
1
1
1
1
will be-a) ─1.96V b) +1.96V
c) ─2.64V d) +2.64V
Barishal Board-2015:
10. According to electrochemical series which one is
correct?
a) Al>Ni
b) Zn>Mg c) Fe>Na d) Cu>Sn
18. For how many minutes 1.2A current should be
passed through silver nitrate solution so that 1.61g
silver is deposited at the cathode?
a) 40min
b) 30min
c) 25min d) 20min
■Answer the next two questions based on the stem
given below:
At 25°C temperature, E°Zn/Zn2+=0.76V; E°Cd2+/Cd =
─0.40V
21. What is the e.m.f of the above cell?
a) 1.16Volt b) 0.76Volt c) 0.40Volt d) 0.36Volt
22. In case of the above cell—
i. Solution of Zn ion can be stored in Cd vessel
20. In case of the above stemii. Zinc acts as anode
i. ‘A’ shows bright light
iii.Cell diagram of the cell is:
ii. ‘B’ shows bright light
Zn(s)|Zn2+(aq)||Cd(s)|Cd2+(aq)
iii. In both the cases heat energy is converted to
Which one is correct?
electrical energy
a) i & ii
b) ii & iii
c) i & iii d) i, ii & iii
Which one is correct?
Sylhet Board-2015:
a) i & ii
b) ii & iii c) i & iii d) i, ii & iii
11. Which one of the followings is used as anode and
21. Order of nonmetallic nature of the metals of the
cathode in H-fuel cell?
stem is-a) Ni
b) Ag
c) Pt d) Graphite
a) Al>Fe>Cr>Mg
b) Mg>Al>Cr>Fe
13. Secondary reference electrode is—
c) Al>Cr>Fe>Mg
d) Fe>Cr>Al>Mg
i. Pt,H2(1atm)/H+(1M) ii. Ag(s),AgCl(s)/HCl(aq)
24. The condition of spontaneity is-iii. Hg(l),Hg2Cl2(s)/KCl(aq)
i. E°cell should be positive
Which one is correct?
ii. ∆G° should be negative
a) i & ii
b) ii & iii
c) i & iii d) i, ii & iii
iii. ∆G° should be positive
19. If 1F charge is passed through molten NaCl,
Which one is correct?
MgCl2, AlCl3 and SnCl4, then which metal will be
a) i & ii
b) i & iii c) ii & iii d) i, ii & iii
deposited at the cathode in higher number of moles?
Comilla Board-2015:
a) Na
b) Mg
c) Al
d) Sn
2. According to the reaction: Al3+ + 3e─→Al, how
Dhaka Board-2016
much charge is needed to extract 9gm of Al metal?
3. Which one of the followings is oxidation-reduction
a) 1F
b) 2F
c) 3F
d) 4F
half cell? a) Pt, Cl2/Cl─
b) Ag, AgCl(s)/Cl─
+
16. The condition of making standard hydrogen
c) Na-Ag/Na
d) Pt/Fe2+, Fe3+
electrode—
4. How much charge is needed to deposite 27g Al?
i. Concentration of solution is 1molL─1
a) 1F
b) 3F c) 13.5F
d) 27F
ii. Pressure of gas is 1atm iii. Temperature is 298K
5. Which one of the followings is used in calomel
Which one is correct?
electrode?
a) i & ii
b) ii & iii
c) i & iii d) i, ii & iii
a) HgCl2 b) Hg2Cl2 c) MnO2 d) NH4Cl
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
24
8. If the standard reduction potential ofs of Cu and Zn
are respectively +0.34 and ─0.76V theni. Cu is stronger oxidant than Zn
ii. if Zn is added to CuSO4 solution, Cu will be
precipitated
iii. Zn will act as anode
Which one is correct?
a) i&ii
b) ii & iii
c) i & iii d) i, ii & iii
17.EZn/Zn2+=+0.76V and EZn/Zn2+=─0.799V; What is
the EMF of the above cell? a) 1.559V b) 1.459V
c) 1.669V d) 2.559V
18. Which one is used as anode with Pt,H2/H+?
a) Zn2+/Zn b) Ag+/Ag c) Cu2+/Cu d) Hg22+,Hg
22. Which one is primary reference electrode?
a) Calomel
b) Gas electrode
c) Hydrogen
d) Oxidation-reduction
24. Which one of the followings can act both as
oxidant and reductant?
a) KI b) H2C2O4 c) Na2S2O3 d) H2O2
Chittagong Board-2016:
>>>>No Questions Came<<<
Rajshahi Board-2016:
6. If 1.0C charge is passed through the solution of
ZnSO4, how much Zn will dissolve at the anode?
a) 34.7g b) 0.000348g c) 32.7g d) 0.0348g
13. Sugar and glucose are—
a) Electrolyte
b) Electronic conductor
c) Non-electrolyte d) Non-metallic conductor
32. Which one is primary reference electrode?
a) Calomel electrode b) Ag/AgCl electrode
c) Zn electrode
d) Hydrogen gas electrode
■Read the stem and answer the next two questionsH2(g)+Cu2+(aq)→2H+(aq)+Cu(s); E°Cell=0.34V
34. Which one is the oxidation reaction at the anode
of the above cell?
a) 2H+ + 2e─ = H2
b) Cu = Cu2+ + 2e─
c) H2 = 2H+ + 2e─
d) Cu2+ + 2e─= Cu
35. What is the electrode potential of E°Cu/Cu2+
electrode?
a) 0.34V b) ─0.34V c) 0.17V d) ─0.17V
Comilla Board-2016:
3. Which one of the followings is used as fuel at the
anode of fuel cell?
a) Hydrogen gas
b) Water gas
c) Oxygen gas
d) CO2-gas
9. If 500mA current is passed through CuSO4 solution
for 1hr, how much copper will be deposited at the
cathode?
a) 5.5222g b) 5.7222g c) 5.8222g d) 5.9222g
19. Which one is used in lead storage battery?
a) HNO3 b) H2SO4 c) HCl d) CH3COOH
■Read the stem and answer the next two questionsAn electrochemical cell ha been constructed by Zinc
and Iron metals. In case of this cell the standard
reduction potentials of Zinc and Iron are ─0.76V and
─0.44V respectively.
22. The cell potential of the above cell is—
a) +0.32V b) ─0.32V c) +1.20V d) ─1.20V
23. In the light of the above mentioned information—
i. zinc salt solution cannot be stored in Iron vessel
ii. The cell reaction occurs spontaneously iii. Zinc
will decay
Which one is correct?
a) i & ii
b) ii & iii
c) i & iii d) i, ii & iii
27. In standard hydrogen electrode the concentration
of acidic solution is—
a) 1.0M
b) 0.1M
c) 0.01M
d) 0.001M
Jessore Board-2016:
12. During electrolysis which one will be discharged
first?
a) Cu2+
b) H+
c) Pb2+
d) Na+
13. Which one is used as electrolyte in lead storage
battery?
a) H2SO4 b) PbSO4
c) PbO
d) PbO2
14. Faraday’s law is applicable in –
i. Metal extraction ii. Electroplating iii. Metal
purification
Which one is correct?
a) i & ii
b) i & iii
c) ii & iii d) i, ii & iii
15. Which one is rechargeable?
a) Lithium battery b) Lithium ion battery
c) Dry cell
d) Alkaline fuel cell
■Read the stem and answer the next two questions(i) M2+(aq) + 2e─ → M(s); E°M2+(aq)/M(s)=0.34V
(ii) N(s)→ N2+(aq) + 2e─; E°N(s)/N2+(aq)= ─0.80V
16. Which cell diagram is correct?
a) M(s), M2+(aq)║ N2+(aq),N(s) b)
M(s),M2+(aq)║N(s), N2+(aq)
c) N(s), N2+(aq) ║ M2+(aq), M(s) d) N(s),
N2+(aq)║M(s),M2+(aq)
17. What is the emf of the above cell?
a) 1.14V b) 0.46V c) ─0.46V d) ─1.14V
Dinajpur Board-2016:
4. Zn(s)│Zn2+(aq)║ Cu2+(aq)│Cu(s)
What is the cathode reaction in the above cell?
a) Zn→ Zn2+ + 2e─
b) Cu2++ 2e─→ Cu
c) Zn2+ + 2e─→Zn
d) Cu→ Cu2+ + 2e─
9. What is the standard reduction potential of Cu?
a) +0.34V b) +0.80V c) +1.30V d) +1.36V
11. In hydrogen fuel cell—
i. Efficiency is 60% ii. Hydrogen gas is used as fuel
iii. The concentration of alkali remains unchanged
Which one is correct?
a) i & ii
b) ii & iii
c) i & iii d) i, ii & iii
23. If 1F charge is passed through CuSO4 solution,
how much copper will be deposited?
a) 23.0g
b) 26.52
c) 31.75g d) 33.68g
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
25
26. Which equation is used to determine the e.m.f of
any cell?
a) Ecell = Eanode(ox) ─Ecathode(ox)
b) Ecell = Ecathode(ox)─ Eanode(ox)
c) Ecell = Eanode(red) ─Ecathode(red)
d) Ecell = Eanode(ox) +Ecathode(ox)
Barishal Board-2016:
■Read the stem and answer the next two questions:
FeSO4 solution has been kept in Zinc vessel. The
standard reduction potentials of Zinc and Iron are
respectively ─0.76V and ─0.44V.
17. The cell potential of the cell produced in stem is-a) +0.32V b) ─0.32V c) +1.20V d) ─1.20V
18. In case of the cell produced in the stem—
i. The Zinc vessel acts as cathode
ii. Concentration of solution is 1M
iii. 1mol substance remains dissolved in 1L soln
Which one is correct?
a) i & ii
b) ii & iii
c) i & iii d) i, ii & iii
22. Which one of the followings is the example of
metal-metal ion electrode?
a) Ag(s)│AgCl(s)│NaCl(aq)
b) Ag(l), Hg2Cl2(s)│KCl(aq)
c) Ag(s)│AgNO3(aq)
d) Pt(s)│Fe2+(aq), Fe3+(aq)
24. Which one of the followings is discharged first?
a) Br─
b) NO3─
c) OH─
d) Cl─
Sylhet Board-2016:
■Answer next two questions based on following stem:
33. For the above cell:
i. E°Cell = E°Zn/Zn2+ + E°Cu/Cu2+
ii. E°Cell = E°Zn/Zn2+ ─ E°Cu/Cu2+
iii. E°Cell = E° Cu2+/Cu + E° Zn2+/Zn
Which one is correct?
a) i & ii
b) i & iii
c) ii & iii d) i, ii & iii
34. If Fe/FeSO4 is used in place of the right half cell
then what will be the cell potential?
a) +1.2
b) ─1.2
c) +0.32 d) ─0.32
13. Which pair of metal is used in electrode?
a) Hg, Au b) Hg, Pt
c) Pt, Au d) Pt, V
20. E°Ag/Ag+ =+1.32V, E°H/H+ = 0V; The cell diagram
constructed by these electrodes will bei. Ag(s)/Ag+(aq)║H+(1M)/H2(g); 1atm, Pt
ii. Ag(s)/Ag+(aq)│H+(aq)/H2(g); 1atm, Pt
iii. Ag(s)/Ag+(aq)║H+/H2(g); Pt
Which one is correct?
a) i & ii
b) ii & iii
c) i & iii d) i, ii & iii
30. If 5amp current is passed through AgNO3 solution
for 60min then how much Ag will be deposited at the
cathode?
a) 8.766 b) 16.812
c) 20.145 d) 24.854
Dhaka Board-2017:
2. Which element is used in semi-conductor?
a) Cu
b) Al
c) Zn
d) Ge
5. In lead cell—
i. Pb foil is anode
ii. Pb, layered by PbO2 is cathode
iii. 30% H2SO4 is used as electrolyte.
Which one is correct?
a) i & ii
b) ii & iii
c) i & iii d) i, ii & iii
17. The cell made by those electrodesa) Zn(s)/Zn2+(aq) b) Fe2+(aq)/ Fe(s)
c) Zn(s)/Zn+(aq)
d) Cu2+(aq)/ Cu(s)
22. Which one of the following batteries is for heart
pacemaker?
a) Lithium ion battery
b) PEM-ion battery
c) Lithium SVO battery
d) Dry cell battery
Chittagong Board-2017:
4. Which one is calomel?
a) HgCl2
b) Hg2Cl2 c) HgF2 d) Hg2I2
5. What amount of electricity is passed through
CuSO4 solution to deposite 1 mole Copper at the
cathode?
a) 1F
b) 2F
c) 3F
d) 4F
6. What is the e.m.f of dry-cell?
a) 1.1 V
b) 1.2 V c) 1.5 V
d) 1.8 V
13. Which one is the anode of dry-cell?
a) NH4C1 b) MnO2 c) Zn d) Carbon electrode
14. Which one of the followings is used as electrolyte
in the salt bridge? a) KC1, KNO3, NH4NO3 b)
KC1, K2SO4, Na2SO4
c) KC1, NH4C1, Na2CO3 d) KC1, NH4C1, NaNO3
15. Which one is above in the reactivity series?
a) Pb
b) Cu
c) Ag
d) Ca
21. Which is used as Cathode in
Pt, H2/H+ (E° = 0.0V)?
a) Zn2+/Zn b) Mg2+/Mg c) Cu2+/Cu d) Fe2+/Fe
22. Lithium ion battery is— i. primary indicator cell
ii. rechargeable battery
iii. used in the preparation of cell phone, Laptop etc.
Which one is correct?
a) i & ii
b) i & iii
c) ii & iii d) i, ii & iii
Rajshahi Board-2017:
7. What is the voltage of dry cell?
a) +0.34V b) +0.76V
c) +1.00V
d) 1.5V
17. Which one of the followings acts as anode?
a) Ag+/Ag
b) Fe2+/Fe
c) Zn2+/Zn
d) H+/H2(g),Pt
19. If 5A current is passed through electrodes for 30s
then charge will be—
a) 60C
b) 95.52C
c) 120C
d) 150C
Comilla Board-2017:
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
26
■Answer next two questions based on following stem:
E°M/M2+ = 0.76 Volt. E°N/N+= ─0.4 Volt
18. In the cell 'M' metal i. is reduced ii. acts as an anode
iii. more reactive than N
Which one is correct?
a) i & ii
b) i & iii
c) ii & iii d) i, ii & iii
19. What is the e.m.f of the cell?
a) ─1.16 V b) ─0.36 V c) + 0.36V d) + 1.16 V
20. Which salt is used in salt bridge?
a) KC1
b) NH4C1
c) NaNO3
d) NaCl
All Board-2018:
5. If 0.1 A current is passed through the M(III)
Sulphate solution 1.0 g M is deposited on cathode.
(Atomic mass of metal M = 40). How many time will
be required to deposit 1.0g metal M?
a) 20s b) 1206s c) 24,125s d) 72,375s
6. In which cases electricity does not produce?
a) Electrolytic cell
b) Lead-storage cell
c) Lithium-ion battery
d) Galvanic cell
Answer next two questions based on following stem:
Zn(s) + FeSO4(aq) → ZnSO4(aq) + Fe(s)
Zn(s) | Zn2+(aq) = + 0.76V Fe(s)|Fe2+(aq) = +0.44V
7. What is cell potential according to stem?
a)─0.42V b) -1.20V c) +0.42V d) +1.20V
[N.B: Correct answer will be +32V]
8. The correct information for the stem’s reaction is—
i. zinc solution can be kept in iron pot
ii. iron solution can be kept in zinc pot
iii. the cell reaction will be spontaneous.
Which one is correct?
a) i
b) ii
c) i & iii d) ii & iii
11. Which one is reduced in lead-storage cell?
a) Pb
b) PbO
c) PbSO4
d) PbO2
Dhaka Board-2019:
3. Which one is the Nernst equation of the cell
reaction: An+(aq) + B(s)→A(s) + Bn+(aq).
n+
n+
A
B
RT
RT
a) Ecell=E°cell ─ ln n+ b) Ecell=E°cell ─ ln n+
nF
nF
B
A
A n+
2.303RT
c) Ecell=E°cell─
log
F
Bn+
Bn+
2.303RT
d) Ecell = E°cell ─
[Ans: b]
log
F
A n+
13. What is the reason of electric conductivity of
liquid NaCl?
a) Free electron
b) Free atom
c) Free ion
d) Free molecule
15. LiCoO2
A + nLi+ + ne─ ; In A compound what
is the oxidation no. of Co?
a) + 4
b) + 3
c) + 2
d) + 1
16. If 1F electricity is passed through the solutions of
A+, B2+ and C3+ ions, then
i. 1 mol A+ is discharged
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
27
3.Which one is the molecular formula of calomel?
a) Hg2Cl2
b) HgCl2 c) Cu2Cl2 d) CuCl2
14. The rechargeable batteries arei. Lead storage battery
ii. Lithium ion battery
iii. Lithium battery
Which one is correct?
a) i & ii
b) ii &iii c) i & iii
d) i, ii & iii
Jessore Board-2017:
15. Which one of the followings is most powerful
reducing agent?
a) Fe
b) A1
c) Li
d) Zn
Dinajpur Board-2017:
4. What is the EMF value of dry cell?
a) +1.5 V b) +1.4 V
c) +1.3V
d) +1.2V
6. Which one is the cell diagram of lead storage cell?
a) Pb/Pb2+║Cu2+/Cu
b) Pb/PbSO4║H2SO4(aq)/PbO2,Pb
c) Zn/Zn2+║Pb2+/Pb
d) Pb/Pb2+║H+/H2
9. Which one of the following is rechargeable?
a) Dry cell
b) Alkaline fuel cell
c) Lithium battery d) Lithium ion battery
22. What is the electrochemical equivalent weight of
'Zn'? a) 3.388×10─4
b) 3.30×10─4
c) 3.29×10─4
d) 3.22×10─4
Barishal Board-2017:
■Answer next two questions based on following stem:
A galvanic cell that contains the following half cells:
Cl2(g)+2e─ → 2CI─ (aq); E° = + 1.36 volt
Cu2+(aq) + 2e─ → Cu (s); E° = + 0.34 volt
5. What is the E°cell of the above cell?
a) +1.7volt
b) +1.02volt
c) ─1.02volt
d) ─1.7volt
6. As per stem which one is the correct cell notation?
a) Cu(s)/Cu+(aq)║Cl2(g)/2Cl─(aq)
b) Pt(s),Cu(s)/Cu2+(aq) ║Cl2(g)/2Cl─(aq),Pt(s)
c) Cu(s)/Cu2+(aq)║Cl2(g)/2Cl─(aq), Pt(s)
d) Cu(s)/Cu2+(aq)║ 2Cl─(aq)/Cl2(g), Pt(s)
7. Which one of the following metal ions will be
deposited more on cathode when 1F electricity is
passed? a) Zn
b) A1
c) K
d) Ca
10. Anodes of fuel cell are—
i. Hydrogen gas ii. Methanol solution iii. Oxygen gas
Which one is correct?
a) i & ii
b) ii & iii
c) i & iii d) i, ii & iii
25. Which one is the primary reference electrode?
a) Calomel electrode
b) Hydrogen electrode
c) Silver Silver Chloride electrode
d) Platinum electrode
Sylhet Board-2017:
16. Which one of the following ions is discharged
first? a) Cu2+
b) H+ c) Au3+
d) Ag+
17. Which one of the following is used as electrode in
fuel cell?
a) Nickel b) Graphite c) Platinum d) Lead
ii. 2 mol B is deposited in cathode
iii. 1/3 mol C3+ is deposited to electrode from the soln
Which one is correct?
a) i & ii b) i & iii c) ii & iii d) i, ii & iii
18. What is the electrochemical equivalent of
Aluminum?
a) 2.8 ×10─5g/c
b) 9.33 ×10─5g/c
c) 1.4 ×10─4g/c
d) 2.8 ×10─4g/c
24. Which one is Oxidation-Reduction electrode?
a) Cu/Cu2+
b) H2/H+(Pt)
c) Pt(S),Fe2+/Fe3+
d) Ag/AgCl, KCl
Chattagram Board-2019:
1. Which metal is present in Calomel?
a) Copper
b) Zinc c) Nickel
d) Mercury
11. How much electricity is needed to generate
1 mol H2 gas in cathode?
a) 1F
b) 2F
c) 3F
d) 4F
19. What is the potential of fuel cell?
a) 1 b) 1.23
c) 2 d) 2.5
Rajshahi Board-2019:
Answer next two questions based on following stem:
16. Farday's Law is applicable—
i. to calculate the charges of electrons
ii. to determine the amount of metal
iii. in case of electrolytic conductor
Which one is correct?
a) i & ii b) i & iii
c) ii & iii d) i, ii & iii
24. What amount of electricity is required to get 1mol
Al from 1 mol A12O3?
a) 1F
b) 1.5F
c) 3F
d) 6F
Jessore Board-2019:
3. Position of Cu in the electrochemical series—
i. Upon Fe
ii. Above Ag
iii. Below H
Which one is correct?
a) i & ii b) ii & iii
c) i & iii d) i, ii & iii
5. How much iron will be deposited if 5A electricity
is passed through FeSO4 solution for 10 minutes?
a) 0.144 g
b) 0.404g
c) 578g
d) 0.868g
Dinajpur Board-2019:
1. At normal temperature H2 can be replaced from
H2O by— a) Ca
b) Mg
c) Zn
d) Pb
5. The cell potential of which Galvanic cell is more?
a) Dry cell
b) Lead acid cell
c) Hydrogen fuel cell
d) Lithium ion cell
11. Which one has more electrochemical equivalent?
a) Ag
b) Cu
c) Fe
d) Cr
2+
17. If Fe/Fe is an anode, which one of the following
may be used as cathode?
a) Zn/Zn2+ b) Mg/Mg2+ c) Al/Al3+ d) Au/Au3+
24. How many grams of chromium will be deposited
when 3A electricity is passed through the chromic
sulphate solution for 6 hours?
a) 11.64
c) 14.21
c) 17.46
d) 21.32
Barishal Board-2019:
1. In Hydrogen fuel celli. efficiency is 65% ii. H2 gas is used as fuel
iii. the concentration of alkali remains unchanged
Which one is correct?
a) i & ii b) ii & iii
c) i & iii d) i, ii & iii
18. Which one acts as an anode among the following
electrodes?
a) Zn2+(aq)/Zn(s) b) Fe2+(aq)/Fe(s)
c) Ag+(aq)/Ag(s)
d) H+(aq)/H2(g), Pt
22. Which ion is discharged easily?
a) Cu2+
b) H+ c) Au3+
d) Ag+
Sylhet Board-2019:
8. Specific gravity of lead storage battery is a) 1.70
b) 1.52
c) 1.39
d) 1.28
22. In case of Lithium ion battery i. Co3+ oxidized to Co4+ during charging
ii. Co4+ reduced to Co3+ at the time of discharging
iii. its voltage is 1.23V
Which one is correct?
a) i & ii b) i & iii
c) ii & iii d) i, ii & iii
13. What is the emf of the cell in the stem?
a) + 1.10V b) + 0.42V c) ─ 1.10V d) ─0.42V
14. According to the stem's cell i. Zn rod acts as an anode
ii. Cu metal is more active than that of Zn
iii. the cell is Zn/Zn2+║Cu2+/Cu
Which one is correct ?
a) i & ii b) ii & iii
c) i & iii d) i, ii & iii
2. Which one is deposited at cathode when electricity
is passed through molten sodium chloride?
a) Sodium metal
b) Hydrogen gas
c) Sodium hydroxide d) Chlorine gas
8. 0.16A electricity is passed through CuSO4 solution
for 40 minutes. What is the number of deposited
copper atom at cathode? [Cu = 63.5]
a) 1.198×1021 number
b) 1.342×1021 number
21
c) 1.546×10 number
d) 1.921×1021 number
Comilla Board-2019:
5. In which half-cell standard hydrogen electrode acts
as a cathode?
a) Zn(s)/Zn2+(aq)
b) Ag(s)/Ag+(aq)
2+
c) Cu(s)/Cu (aq)
d) Au(s)/Au3+(aq)
8. What is the EMF of the following cell at standard
state? Sn(s)/Sn2+(aq)||H+(aq)/H2(g) (1atm, Pt);
Here; E°Sn2+(aq)/Sn(s) = ─ 0.14 V
a) ─0.14V b) ─0.07 V
c) +0.07 V d) +0.14 V
‘One day everybody has to die’
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
28
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
29
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
30
Mohammad Nizamuddin, Assistant Professor of Chemistry, Cantonment English School & College, Cell: 01815351290
31