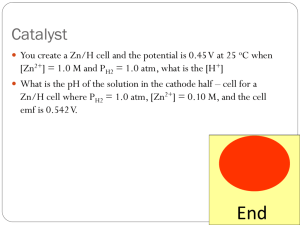
Galvanic Cell Demonstration: Zn/Zn2+ and Cu/Cu2+
advertisement

NCSU – Dept. of Chemistry – Lecture Demonstrations Electrochemistry Galvanic Cell (Zn2+/Zn with Cu2+/Cu) Description: A galvanic cell is constructed using Zn2+/Zn and Cu2+/Cu couples with a NaCl salt bridge. Materials: 1 M CuSO4 1 M ZnSO4 Zn and Cu strips Voltmeter Connecting wires Salt bridge strips Procedure: For large lecture halls, project demonstration using a document camera. 1. Pour 1 M CuSO4 and 1 M ZnSO4 solutions in separate containers (or use setup in Dabney 114). Place the Zn electrode in the ZnSO4 solution and the Cu electrode in the CuSO4 solution. 2. Connect the black wire (anode) to the Zn electrode and the red wire (cathode) to the Cu electrode. The voltmeter will not show any reading until the circuit is completed by the salt bridge (filter paper strip soaked in saturated NaCl solution). Make sure the salt bridge makes contact with both solutions. Discussion: Due to their respective reduction potentials, a spontaneous redox process occurs when the zinc electrode is connected to the negative terminal and the copper electrode is connected to the positive terminal of the voltmeter. The spontaneous flow of electrons from anode to cathode generates a current with a voltage near the theoretical Eocell for these couples (1.10 V). Anode Cathode Zn → Zn2+ + 2 e1Cu2+ + 2 e1- → Cu Eored (V) - 0.76 + 0.34 Safety: Wear proper protective equipment including gloves and safety glasses when preparing and performing this demonstration. NCSU – Dept. of Chemistry – Lecture Demonstrations Electrochemistry Disposal: Copper and zinc solutions can be reused as long as no contamination occurred. Electrodes should be rinsed, dried and cleaned by scrubbing with steel wool. References: Shakhashiri, B. Z. In Chemical Demonstrations: A Handbook for Teachers of Chemistry; The University of Wisconsin Press: 1992; Vol. 4, p 101-106. Video : http://www.youtube.com/watch?v=A0VUsoeT9aM (animation) http://www.youtube.com/watch?v=0oSqPDD2rMA (animation)