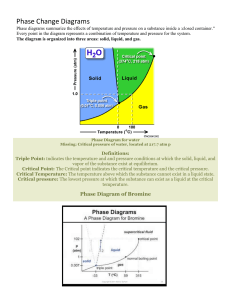
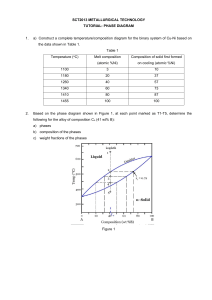
Phase Diagrams: Materials Science Presentation
advertisement

Lecture 17 Phase Diagrams Department of MME BUET, Dhaka Introduction to Phase Diagrams Some properties of materials are functions of their microstructures, and, consequently, of their thermal histories. The microstructures are controlled by the composition of the material and how it is processed. So it is important that we must know about the structure of a material that has been developed during its manufacture, and the method of controlling (and/or modifying) the structure to enhance its properties. Phase diagram is an important tool for materials scientists that tells which phases are stable in a system under specified conditions (e.g. of temperature, overall composition, pressure) Even though most phase diagrams represent stable (or equilibrium) states and microstructures, they are nevertheless useful in understanding the development and preservation of nonequilibrium structures and their attendant properties; The understanding of phase diagrams for alloy systems is extremely important Because, there is a strong correlation between microstructure and mechanical properties, and the development of microstructure of an alloy is related to the characteristics of its phase diagram In addition, phase diagrams provide valuable information about melting, casting, crystallization, and other phenomena Phase diagrams are also known as the equilibrium diagrams or constitutional diagrams. Phase diagram is basically a map that presents the domains of stability of phases and the limits of stability of phases in a graphical form. Reading the map will tell you, at the state when it comes to equilibrium, 1. 2. 3. what phases are present, the state of those phases, and the relative quantities of each phase. Reading a phase diagram will also tell what phase transformations we can expect when we change one or more parameters of the system (T, P, X). Limitations of Phase Diagrams ❑ Rate of phase transformations is missing. ❑ TTT (Temperature-Time-Transformation) diagrams complement the phase diagrams. ❑ Phase diagram gives information only on the constitution of alloys but not on the structural distribution of the phases. ❑ Phase diagram show only the equilibrium state whereas alloys in practical use are rarely in equilibrium. Definitions and Basic Concepts Components ❑ Chemically recognisable species that are mixed to form the alloy. Pure metal or compounds of which alloy is made. Solute and solvent, which are also common terms. In Brass: Cu, Zn (element) In steels: Fe, C (element) In ceramics: SiO2, Al2O3 (compound) ❑ Binary alloy contains 2 components, ternary 3, etc. System ❑ relate to the series of possible alloys consisting of the same components, but without regard to alloy composition (e.g., the iron–carbon system). ❑ An alloy is a substance that has metallic properties and is composed of two or more chemical elements, of which at least one is a metal. Hence, An alloy is a combination of metals or of a metal and another element. ❑ Alloying elements are deliberately introduced into a metal to enhance properties especially, mechanical properties. Yield strength is defined as the stress at which a material ceases elastic deformation and begins plastic deformation Property 60% Ni Monel Cu Wt % Nickel Tensile strength Yield Strength % Elongation Electrical Conductivity Ni Direction Up Up Down Down tensile strength: maximum load that a material can support without fracture when being stretched, divided by the original cross-sectional area of the material. An alloy is distinct from an impure metal in that, with an alloy, the added elements are well controlled to produce desirable properties, while impure metals such as wrought iron are less controlled, but are often considered useful. Note the difference Alloy systems Alloying element and impurity element Alloy and alloy system Binary system (Fe-C system, Cu-Zn system) Ternary system (Fe-C-Mn system, Al-Si-Mg system) o The mechanical properties of alloys will often be quite different from those of its individual constituents. o A metal that is normally very soft (malleable), such as aluminium, can be altered by alloying it with another soft metal, such as copper. o Although both metals are very soft and ductile, the resulting aluminium alloy will have much greater strength. SOLUBILITY LIMIT ❑ For many alloy systems and at some specific temperature, there is a maximum concentration of solute atoms that may dissolve in the solvent to form a solid solution; this is called a solubility limit. ❑ The addition of solute in excess of this solubility limit results in the formation of another solid solution or compound that has a distinctly different composition. Phase ❑ A phase is a homogenous, physically distinct and mechanically separable portion of the material with a given chemical composition and structure. What and how many phases materials possess? Every pure material is considered to be a phase. Solid, liquid, or gas, (and plasma)? Is it possible to have more than one solid phases? Iron, being an allotropic material, has more than one solid phases: ❑ When iron first freezes from its liquid state, it is BCC (d-iron) ❑ As it cools it changes to FCC (g-iron) ❑ Upon further cooling it changes to BCC (a-iron) Equilibrium state and Metastable state A system is at equilibrium if, at constant T, P and X, the system does not change with time. The equilibrium state always has the minimum free energy. ❑ Equilibrium state requires sufficient time to achieve. When this time is too long (due to slow kinetics), another state along the path to the equilibrium may appear to be stable. This is called a metastable state. ❑ A system at a metastable state is trapped in a local minimum of free energy, which is not the global one. Phase Equilibrium A system is at equilibrium if its free energy is at a minimum under some specified combination of temperature, pressure, and composition A change in temperature, pressure, and/or composition for a system in equilibrium will result in an increase in the free energy and in a possible spontaneous change to another state whereby the free energy is lowered. The term phase equilibrium, often used in the context of this discussion, refers to equilibrium as it applies to systems in which more than one phase may exist. Phase equilibrium is reflected by a constancy with time in the phase characteristics of a system. Degrees Of Freedom, F • The degrees of freedom is essentially the number of independent variables (that can be varied independently without changing the number and state of phases at equilibrium), both internal (composition of phases) and external ones(temperature, pressure etc.) whose values must be specified in order to define completely the state of the system. • Factors (variables) that might come in to an equation of state: – – – – – – – – Number of components Number of Phases Composition of the phases Amount of phases Overall composition of the alloy Temperature Pressure Volume Gibb’s Phase Rule: F = C–P+2 F = # degrees of freedom C = # components P = # phases 13 Classification of Phase Diagrams One component (unary) phase diagrams ❑ Also known as P-T diagrams. ❑ The simple case is Water. How many single-phase regions? How many two-phase regions? Is there any three-, or more-phase regions? Unary phase diagram of water This one-component phase diagram (or unary phase diagram) is represented as a two dimensional plot of pressure (ordinate, or vertical axis) versus temperature (abscissa, or horizontal axis). Most often, the pressure axis is scaled logarithmically. Two-component (binary) phase diagrams ❑ How does mixing of A into B effect the bond energies and the melting temperature of the resultant alloy? ❑ Interaction of A and B resulted three bonds: A-A, B-B and A-B bonds. ❑ Example: Copper - Nickel, Silicon - Germanium ➔ Completely miscible/soluble phase diagrams Ge-Si phase diagram (completely miscible) Humayun Kabir, Dept of MME, BUET Ni-Cu phase diagram (completely miscible) Working with Binary Phase Diagrams Most Important Information: Overall composition of the alloy Liquidus and solidus temperatures Limits of solid solubility Identification of equilibrium phases at any given condition Chemical composition of phases at any temperature Amount of phases (relative amount) at any temperature Phase Diagram Nomenclatures Concentration/Overall Composition of alloy ❑ Relative amounts of each constituent ❑ It is the horizontal axis in all binary phase diagrams ❑ The scale can be in weight %, atomic % or mole % Liquidus temperature ❑ Start of solidification (or, end of liquification) temperature Solidus temperature ❑ End of solidification (or, start of liquification) temperature Liquidus Liquid Freezing range Liquid + Solid Solidus Solid X % Y added Y Phase diagram with complete solubility of one component into another The copper–nickel phase diagram The liquid L is a homogeneous liquid solution composed of both copper and nickel. The Alpha phase is a substitutional solid solution consisting of both Cu and Ni atoms and having an FCC crystal structure. The copper–nickel phase diagram ❑ At temperatures below about 1085ºC, copper and nickel are mutually soluble in each other in the solid state for all compositions. ❑ This complete solubility is explained by the fact that both Cu and Ni have the same crystal structure (FCC), nearly identical atomic radii and electro-negativities, and similar valences, as discussed previously. ❑ The copper–nickel system is termed isomorphous because of this complete liquid and solid solubility of the two components. Chemical composition of Phases ❑ It is the chemical composition of each phase in the system. ❑ In a system having more than one phase, each phase will have a unique chemical composition which will be different from each other, and will also be different from the overall composition. ❑ Not to be confused with overall composition of the alloy. Relative amounts of Phases ❑ When a system contains more than one phases, then it is the amount of each phase relative to overall amount of the alloy. ❑ Depends on temperature and composition of the alloy. ❑ Not to be confused with composition of phases. Composition of each phase Phase diagram rule #2: Tie Line Rule If we know C0, then we can tell the composition of each phase at any temperature. In a system having more than one phase, each phase will have a unique chemical composition which will be different from each other, and will also be different from the overall composition of the system. Not to be confused with overall composition. tie line At TA: L phase only CL = 35%Ni At TB: L and a phases CL = Cliquidus = 32%Ni Ca = Csolidus = 43%Ni At TD: a phase only Ca = 35%Ni Relative amount of each phase Phase diagram rule #3: Lever Rule If we know C0, then we can tell the weight fraction of each phase at any temperature. Consider C0 = 35 wt.%Ni At TA, only L phase WL = 100 wt.% At TD, only a phase Wa = 100 wt.% At TB, both L and a phases Lever Rule 100 S 100 (43-35) WL = = = 73 wt.% R+S 43-32 100 R 100 (35-32) Wa = = = 27 wt.% R+S 43-32 It is the relative amount (as in kg) of each phase in the whole alloy. Not to be confused with composition (i.e., %A and %B) of the alloy. To summarise: Finding the composition in a two phase region: 1. 2. 3. Locate composition and temperature in the diagram. In the two phase region, draw a tie line at the given temperature. The liquid composition will be on the liquidus line and the solid composition will be on the solidus line. Note the intersections with phase boundaries. Read compositions at the intersections. Finding the amounts of phases in a two phase region: 1. 2. 3. Locate composition and temperature in the diagram. In the two phase region, draw a tie line at the given temperature. Fraction of a phase is determined by taking the length of the tie line to the phase boundary for the other phase, and dividing by the total length of tie line.