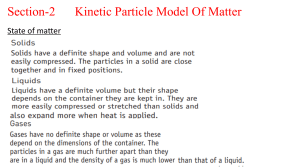
1.1 Matter and Energy Faster moving particles have more kinetic energy (gas >liquid>solid) Temp is a measure for average kinetic energy of particle of matter. (gas temp > liquid temp> solid temp) Heat= kinetic energy transferred from matter at higher temp to a lower temp. Matter has potential energy as a result of position/composition. o High potential energy = less stable o Lower potential energy = more stable 1.2 Units and Measurements Prefix unit = (10x or –x) X (base unit) 1ml = 1cm3; 1L = 1000 cm3 Volume of object can be determined by measuring volume of water displaced. o Volume object = Volume Final – Volume initial 1.3 Significant Figures in Measurements and Calculation Last number measured is uncertain and estimated digit.