Two-Component Liquid Systems: Phenol-Water Miscibility
advertisement

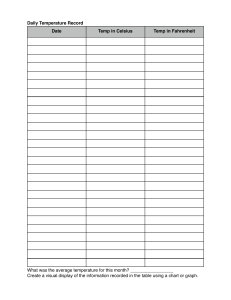
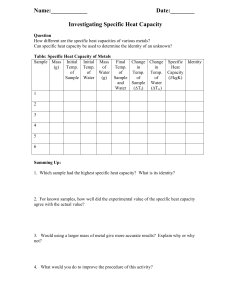
Lab-2Two component systems containing liquid phases From the previous lab. We learn that pharmaceutical preparations can be formulated either as true solutions or dispersions (colloidal or coarse) which they contain more than one phase. Phase: -is homogeneous physically distinct portion of the system which is separated from other parts of the system by boundary surfaces. (e.g. water & its vapor is one component two phase system) Component: is a substance in the system with a defined chemical structure. A phase may contain one or more component. Miscibility: refers to the ability of liquid to completely dissolve in another liquid solution. As we know alcohol & water are miscible in all proportions, while water & mercury are completely immiscible regardless the amount of each. Between these two extremes lie a whole range of system which exhibit a partial miscibility (or immiscibility) such as water & phenol, as their miscibility affected by two factors conc. & temp. To illustrate the effect of conc. & temp. we prepare the following conc. of phenol in water by % weight as the total wt. is (10 gm) 2%,7%,9%,11% ,24%,40%,63% and 70% (w/w) (e.g. 2% 0.2 gm phenol + 9.8 gm H₂O) *1)Effect of the conc.:-We record which one is one phase & which is two, at room temp. -As the conc. Increase, deeper color appears. -phase B lies below phase A bec. Phenol has higher density than water *2) Effect of the temp.:As the temp. increase, miscibility increase ( no. of the samples of the two phases decrease ) To see the effect of temp. & conc., we draw graph paper of temp. versus conc. -Binodal curve:- is the curve that separates two phase area from one phase area . -Tie line :- is the line drawn across the region of two phases (conjugate phases ) as each temp. has its own tie line. It is always parallel to the base line in two component system. Conjugated phases: are two phases existing together in equilibrium at certain temperature and has a constant composition. -Upper consolute temperature (UCT): is the temp. above which all the systems existing as one phase, also is called critical solution temperature. Ex: Water & phenol system it is 66.8 C as all combinations above this temp. is completely miscible & give one phase system Mass ratio:-is the relative amount by wt. of conjugate phase ,it depends on the position in tie line & temp. EX: calculate the mass ratio of 40% w/w phenol in water if you know total weight =10 gm? Tie line 11%---63% at 25C. Mass ratio = 𝑊𝑡. 𝑝ℎ𝑎𝑠𝑒 𝐴 𝑊𝑡. 𝑝ℎ𝑎𝑠𝑒 𝐵 63−40 Mass ratio= 40−11 23 = 29 23+29=52 total parts of the system Part 23 X total part 52 29 100 X= 44.23% part A x X= 55.76% part B total 52 100 -properties of the tie –line in two component systems:1-It is parallel to the base line 2-All systems prepared along the tie line at equilibrium separated into two conjugate phases of constant composition (e.g. at 25 C any conc. At the tie line contain two phases (A)&(B)of constant composition7% (A)& 70% (B) of phenol). Procedure: 1. Prepare the following percent W/W phenol/water (10 gm total) 2%,7%,9%,11% ,24%,40%,63%,70%. 2. Put test tube in a fixed temperature in water bath (25 C0) or (left test tube at room temp.) and keep it for 10 minutes at that temp. 3. Take the test tubes out and before their temp has changed record which one has 2 phases and which has one phase. 4. Repeat the work at higher temp using the following temp.40C0, 50C0, 70C0. 5. Draw a curve temp verses concentrations showing your 2 phases area and one phase area in the curve. 6. Draw tie line for each temp. 7. Take 40% W/W for example to find the mass ratio and the composition of each phase at different temp. 8. Mention the upper consulate temp.