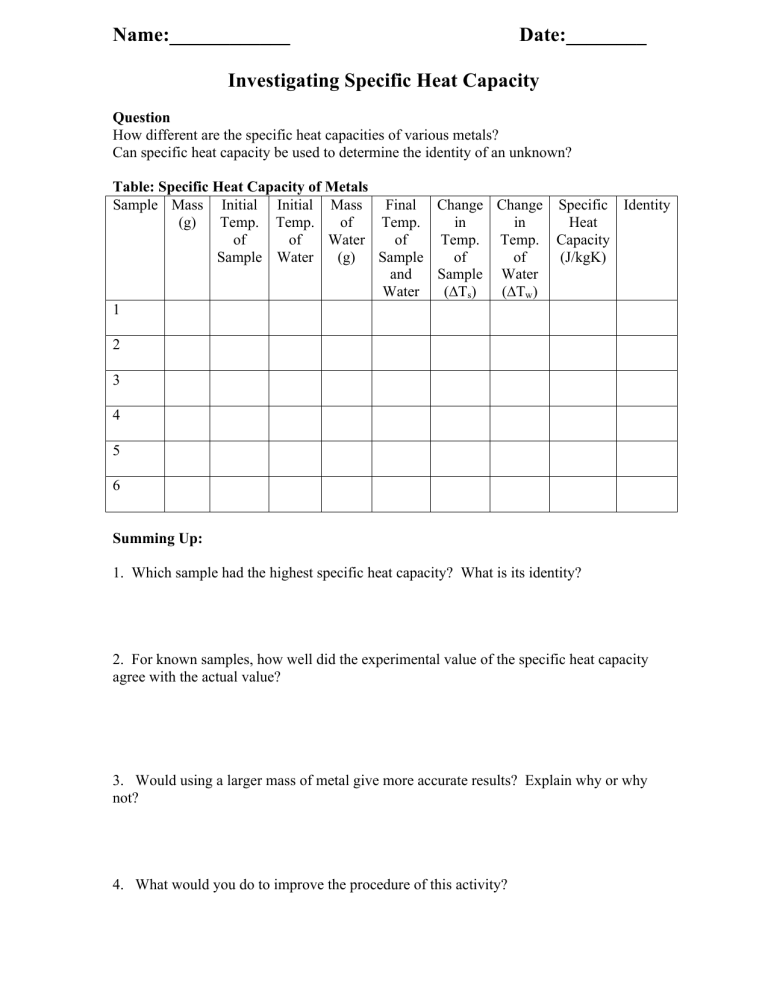
Name:____________ Date:________ Investigating Specific Heat Capacity Question How different are the specific heat capacities of various metals? Can specific heat capacity be used to determine the identity of an unknown? Table: Specific Heat Capacity of Metals Sample Mass Initial Initial Mass Final Change Change Specific Identity (g) Temp. Temp. of Temp. in in Heat of of Water of Temp. Temp. Capacity Sample Water (g) Sample of of (J/kgK) and Sample Water Water (∆Ts) (∆Tw) 1 2 3 4 5 6 Summing Up: 1. Which sample had the highest specific heat capacity? What is its identity? 2. For known samples, how well did the experimental value of the specific heat capacity agree with the actual value? 3. Would using a larger mass of metal give more accurate results? Explain why or why not? 4. What would you do to improve the procedure of this activity?