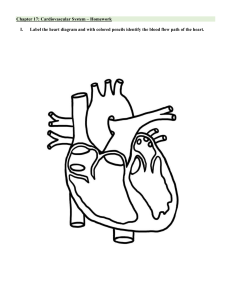
CHAPTER 1 BASIC INFORMATION I. II. Introduction to Internal Medicine III. Normal Laboratory Values and Conversion Factors 1. Complete Blood Count 2. Blood Chemistry 3. Urine Studies 4. Equivalent Values IV. Intravenous Fluids 1. Intravenous Fluids and Common Indications V. Commonly Used Drips 1. Formulation and Computation of Basic Drips 2. Other Commonly Used Drips Core Skills in Internal Medicine 1. Electrocardiography 2. Chest Radiograph Interpretation 3. Arterial Blood Gas Interpretation 4. Thoracentesis 5. Paracentesis 6. Foley Catheter Insertion 7. Intravenous Line Insertion 1 SECTION 1 INTRODUCTION TO INTERNAL MEDICINE I. INTRODUCTION Internal Medicine (IM) can be quite overwhelming because of the complexity of cases and long work hours. Despite these inherent toxicities, it remains one of the most rewarding fields in Medicine. Students and practitioners alike enjoy the intellectual stimulation and experience of translating theoretical knowledge into direct patient care. As basic IM principles cannot be dissociated from the cases we encounter, it is imperative for every practitioner to acquire the core competencies and skills of an internist. The approach to patient encounter and chart writing are discussed in the succeeding parts. II. HISTORY AND PHYSICAL EXAMINATION Complete history and physical examination are central to hypothetico-deductive reasoning in clinical medicine. Starting from the chief complaint, problems are elicited from the in chronological order. After completing the details for acute complaints, probe into the patient’s past medical history, including present medications and pre-morbid functional capacity. Diseases in the family such as hypertension, diabetes, heart disease, early cardiac death and other heredofamilial diseases should be elicited as part of the family medical history. History of allergic reactions to drugs and food should always be elicited. Dietary habits, smoking history, alcohol intake and illicit drug use should also be included in the personal and social history. Likewise, female patients should be asked about details on menstruation and pregnancy. The comprehensive history is followed by the systematic physical examination (PE). This starts with a general survey followed by checking the patient’s vital signs. Permission should always be asked from the patient before doing any maneuver, especially the more intrusive ones. A complete PE is carried out with special focus on certain procedures pertinent to the identified problems of the patient. III. WRITING THE ORDERS With the information obtained from the history and PE, a prioritized problem list is then created, with the most urgent conditions listed first. Based on the problem list, the management list is then outlined. The orders for the patient usually contain the following: Diet Fluids and Drips Monitoring Diagnostics Therapeutics Transfusions Dietary preparations (i.e., general liquids, soft diet, full diet) and specific dietary prescriptions (i.e., low-salt, low-fat, low-purine, DAT) Main IV lines (i.e., plain saline, D5-containing fluids) and side drips (i.e., vasopressors, electrolyte solutions) BP, HR, RR, temperature, peripheral O2 saturation, neurologic vitals, etc.) Frequency by which these parameters are checked (i.e., q hourly, q4h, q shift) Prioritized list of diagnostic procedures such as imaging, blood tests and special procedures Medications with corresponding doses, frequency of dosing, duration and side effects to watch out for Blood products, the amount to be transfused, rate of transfusion and interval between transfusions Pre-medications and side effects to watch out for Anticipatory measures: diuretics for possible congestion, anti-pyretics for febrile transfusion reactions 2 DATE/TIME 6/10/2015 7:30am PHYSICIAN’S ORDER SHEET Gen Med Diagnosis: Community acquired pneumonia, moderate risk Hypertension, stage I, controlled Diet: Low salt, low fat diet; limit oral fluid intake <1.5L day IVF: PNSS 1L x 10 hours Side drip1: MgSO4 2g in 250cc D5W x 24 hours Dx: Chest X-Ray (PA and lateral views) Complete blood count Tx: 1. Ceftriaxone 2g IV q24h 2. Azithromycin 500mg/tab 1 tab OD for 3 days 3. Losartan 50mg/tab 1 tab PO OD Monitor VS q4 with temp and O2 sat Monitor I&O q shift and record Refer to dietary for dietary prescription and advice Watch out for desaturation and BT reaction Jaime Aherrera MD Lic. No. 123456 IV. PRESENTING THE CASE A. General data – begin with the name, followed by the age, sex, chief complaint, reason for admission, and date of admission or referral “Juan dela Cruz, 28 years old male, admitted yesterday morning for dyspnea.” B. History of present illness – review of systems and pertinent information from the past medical, family medical, personal/social, and obstetric (if applicable) histories C. Significant diagnostic findings and their interpretations, including pertinent normal findings to rule out the differentials being considered by the team D. Hospital course – emphasize the developments or important events that happened to the patient E. Case summary – two or three sentences F. Assessment/problem list G. Plan – based on the assessment/problem list; detailed and specific. Orders for the patient should have their bases and the expected laboratory findings or trends should be known H. Entertain clarifications or questions from the audience 3 SECTION 2 CORE SKILLS IN INTERNAL MEDICINE ELECTROCARDIOGRAPHY I. THE STANDARD 12-LEAD ELECTROCARDIOGRAM (ECG) A. Limb leads Standard limb (Bipolar) leads: I, II, III Augmented limb (Unipolar) leads: aVF, aVR, aVL B. Chest leads V1 II. 4th ICS, right parameter border V2 4th IC, left parasternal border V3 Between V2 and V4 V4 5th ICS, left midclavicular line V5 5th ICS, left anterior axillary line V6 5th ICS, left midaxillary line THE P-QRST WAVES, SEGMENTS AND THE CARDIAC CYCLE 4 III. BASIC STEPS IN ECG READING Step 1: Determine rate Step 2: Determine rhythm Step 3: Measure intervals Step 4: Determine axis Step 5: Look for chamber enlargements Step 6: Check for arrhythmias and other abnormalities STEP 1: DETERMINE HEART RATE Regular Rhythm Heart Rate = 1500_____________ # of small squares from R to R Irregular Rhythm Heart Rate = # of QRS complexes within 30 large boxes x 10 STEP 1: DETERMINE RHYTHM Regular Sinus Rhythm Rhythm is determined by the sinus node, which fires at 60-100 bpm P-wave is normally upright in lead II (and usually in leads I, aVL and aVF) Each p-wave is followed by a QRS complex, and each QRS complex is preceded by a p-wave The distances between the R-R intervals should be equal Sinus Tachycardia and Bradycardia Tachycardia: HR >100bpm Bradycardia: HR <60bpm Sinus Arrhythmia SA node discharges irregularly (sinus node rate varies with the respiratory cycle) Rate: normal Rhythm: varies with respiration, variation in the P-P interval and R-R interval >120 msecs P-waves, PR interval and QRS: normal 5 STEP 3: MEASURE INTERVALS Normal Values Wave/Interval P-wave PR interval QRS duration QT interval (QTc) Description Atrial depolarization Conduction within the AV node Ventricular activation Ventricular activation and recovery Normal Values < 0.12 sec or <120 msec (<3 small boxes) 0.12-0.20 sec or 120-200 msec (3-5 small boxes) < 0.11-0.12 sec or <110-120 msec (<3 small boxes) 0.35-0.43* (males) and 0.45* (females) *may vary depending on reference *Source: Kasper DL, et al. Harrison’s Principle of Internal Medicine, 19th edition Corrected QT-interval (QTc) using the Bazett’s formula Done to adjust for abnormal heart rates (HR <60 or >100 bpm) QT actual Corrected QT interval = R-R interval in sec STEP 4: DETERMINE AXIS Computation of Frontal Axis Deduct negative deflections form positive deflections in QRS complexes to derive the values for leads I and aVF. If lead I is a negative integer, subtract the computed axis from 180 to get the final axis. Note that the value for aVF in the denominator is the absolute value, while that in the numerator takes the sign (positive or negative) into consideration. This is why a predominantly negative deflection in aVF will make the axis negative. ____90 aVF____ Axis = | I | + | aVF Interpretation Normal Axis -30o to 100o Right Axis Deviation (RAD) 100o to 180o Left Axis Deviation (LAD) -30oto -90o Extreme Axis Deviation -90o to -180o “Eyeballing” method – can be used to estimate axis INTERPRETATION Normal LEAD I QRS pointing UP LEAD aVF QRS pointing UP Left Axis Deviation QRS pointing UP QRS pointing DOWN Right Axis Deviation QRS pointing DOWN QRS pointing UP Extreme Axis Deviation QRS pointing DOWN QRS pointing DOWN STEP 5: LOOK FOR CHAMBER ENLARGEMENTS A. Atrial Enlargement Right Atrial Enlargement (RAE) Left Atrial Enlargement (LAE) Peaked P-wave with > 2.5mm amplitude (> 2.5 small boxes) in leads II, III or aVF “P-Pulmonale” or peaked P-wave from pulmonary causes Broad P-wave (> 120ms or > 3 small boxes) Biphasic P wave in V1 with a broad negative component Often notched P-wave in one or more limb leads “P-Mitrale” or M-shaped P-wave 6 B. Left Ventricular Hypertrophy (LVH) SOKOLOW-LYON CRITERIA [S in V1] + [R in V5 or v6] > 35 mm (>35 small boxes) OR CORNELL CRITERIA S in V3 + R in aVL: Female > 20mm Male > 28mm R in aVL>11mm C. Right Ventricular Hypertrophy (RVH) Relatively tall R wave in lead V1 (R > S wave) with right axis deviation R in V1 > 0.7mV R/S in V1 > 1 with R > 0.5 mV R/S in V5 or V6 < 1 IV. ARRYTHMIAS JUNCTIONAL AND IDIOVENTRICULAR RHYTHMS A. Junctional (Atrioventricular) Rhythm Pacemaker: AV junction with a ventricular rate of 40 to 60 bpm P wave: may appear before, after, or buried within the QRS complex Rhythm (RR-interval): regular QRS complex: narrow (<0.12 sec) B. Idioventricular Rhythm Pacemaker: Hiis-Purkinje system (HPS) with a ventricular rate between 15 to 40 bpm P wave: absent Rhythm (RR interval): regular QRS complex: wide (>0.12 sec) 7 DISORDERS OF SINUS RHYTHM A. Sinus Pause Temporary cessation of sinus node activity May be synonymous with sinus “arrest” – which pertains to a prolonged sinus pause (definition is arbitrary) Difference from sinus exit block: the supposed P-P interval of the dropped beat is not a multiple of the normal P-P interval B. Sinus Exit Block Failure of impulse transmission No visible P-QRS-T complex for >1 cycle, wherein the P-P interval of the pause is a multiple of the normal P-P interval (differentiating it from sinus pause) ATRIOVENTRICULAR (AV) BLOCKS A. First Degree AV Block Prolonged PR interval (>0.20 sec or >5 small boxes) P-wave is followed by a QRS complex B. Second Degree AV Block, Mobitz Type I (Wenckebach) PR interval progressively lengthens, then the impulse is blocked (P is not followed by QRS, resulting in a dropped beat) 8 C. Second Degree AV Block, Mobitz Type II PR intervals of conducted beats are constant in length, however, beats are dropped with no warning PR intervals may be normal or prolonged D. High Grade AV Block 2 out of every 3 or more impulses from the atria are blocked by the AV node and fail to reach the ventricles PR intervals are constant (in contrast to complete heart block) E. Third Degree (Complete) AV Block P and QRS waves occur regularly but are independent of each other No consistent relationship between atrial and ventricular activity (AV Dissociation) PP intervals and RR intervals are constant 9 ATRIAL ARRHYTHMIAS A. Premature Atrial Contractions (PAC) Premature P-waves (earlier than the next expected sinus P-wave) P-wave has a different morphology compared to the sinus P-wave since this P-wave is coming from a different atrial focus QRS is usually narrow B. Atrial Fibrillation (AF) Description: Rapid, erratic electrical discharge from multiple atrial ectopic foci Rate: atrial rate >350 bpm; ventricular rate varies Rhythm: irregularly irregular P-waves: no discernable P-wave QRS: usually normal C. Atrial Flutter Description: Re-entrant circuit within the atria, with variable conduction of impulses through the AV node to the ventricles Rate: atrial rate is 250350 bpm; ventricular rate varies Rhythm: variable (depending on conduction) P-waves: saw-tooth appearance QRS: usually normal D. Wandering Pacemaker Description: impulses originate from different foci in the atrium Rate: normal Rhythm: irregular P-waves:> 3 different forms PR interval: variable QRS: normal 10 E. Multifocal Atrial Tachycardia (MFAT) Rate: Fast; Irregular atrial rate (> 100) Rhythm: irregular P-wave: >3 different forms PR interval: variable QRS: normal SUPRAVENTRICULAR TACHYCARDIA (SVT) Arrhythmia has such a fast rate that the P waves may not be seen Rate: 150-250 bpm Rhythm: regular P waves: frequently buried in preceding T waves QRS: normal, but may be wide if abnormally conducted through ventricles (aberrant conduction) Atrioventricular Nodal Reentrant Tachycardia (AVNRT) Most common form of SVT Narrow QRS tachycardia with a short RP interval – P-waves buried in the QRS complex May have a “pseudo-S” wave (which is actually a retrogradely conducted P wave) in inferior leads or “pseudo-R prime” in V1 VENTRICULAR ARRHYTHMIAS Wide QRS tachycardias (>120 ms or 3 small squares): usually ventricular in origin Differentials for wide QRS tachycardia o Ventricular tachycardia (VT): more common o Supraventricular tachycardia (SVT) with aberrancy When faced with a wide-complex tachycardia and the morphology is in question, it is safer to treat as ventricular tachycardia (the more life-threatening differential) A. Premature Ventricular Contractions (PVC) Prematurely occurring QRS complex which is wide and bizarre-looking Usually no preceding Pwave T wave opposite in Bigeminy: PVCs alternate with sinus beats deflection to the QRS 11 complex Trigeminy: PVC occurs after every 2 sinus beats Couplet: two successive PVCs (if three successive PVCs, it is already considered unsustained VT) B. Ventricular Tachycardia (VT) 1. VT According to Morphology a. Monomorphic Ventricular Tachycardia Rapid, bizarre wide QRS complex (appearance of all the beats match each other in each lead) No P-wave Ventricular focus produces a rapid sequence of PVC-like wide ventricular complexes b. Polymorphic Ventricular Tachycardia (Torsades de Pointes) Beat-to-beat variations in appearance Baseline rhythm demonstrates long QT interval Presents with an oscillating pattern mimicking the “turning of the points” stitching pattern 2. VT According to Duration a. Sustained: ventricular tachycardia that lasts for more than 30 seconds b. Non-sustained: ventricular tachycardia that self-terminates within 30 seconds (presence of at least >3 successive PVCs is considered VT) 3. VT Based on Symptoms a. Pulseless VT: no effective cardiac output (no pulse, no BP) b. Unstable VT: with pulse, but unstable BP cardioversion defibrillate (treat as ventricular fibrillation) 12 C. Ventricular Fibrillation (VF) Associated with coarse or fine chaotic undulations No P-wave No true QRS complexes PACEMAKER RHYTHM A. Ventricular Paced Rhythm RR interval is regular QRS complex is wide with an LBBB morphology Pacemaker spike (“blip”) is followed by a wide QRS complex (good capture) B. Atrial Paced Rhythm Atrial pacing appears on the ECG as a single pacemaker stimulus followed by a P wave PR interval and configuration of the QRS complex are similar to those seen in a sinus rhythm C. Dual Pacemaker (Atrial and Ventricular) 13 V. OTHER ABNORMAL FINDINGS ISCHEMIA Findings suggestive of ischemia(should be in 2 or more contiguous leads) ST segment depression > 1mm (> 1 small box) Deep T-wave inversions > 5 mm (> 5 small boxes) For example, if there are ST segment depressions of >1mm in lead V5 and V6: then we can say that there is lateral wall ischemia. If ST segment depressions occur in V3 to V6: then we can say there is anterolateral wall ischemia. The Contiguous Leads II, III, aVF I, aVL V1, V2 V3, V4 V5, V6 V1 – V3 V3 – V6, I, aVL V5, V6, II, III, aVF Almost all leads V3R, V4R (right-sided leads) Inferior wall High lateral wall Septal wall Anterior wall Lateral wall Anteroseptal wall Anterolateral wall Inferolateral wall Diffuse, massive Right ventricular wall INFARCTION A. Findings suggestive of injury or infarction Significant ST elevation:manifestation of myocardial necrosis; the earliest sign of acute infarction > 1 mm ST elevation in contiguous limb leads, or > 2 mm ST elevation in contiguous chest leads B. Pathologic Q-Waves Indicate myocardial necrosis Significant Q-wave: > 0.04 secs duration and > 25% of the R wave amplitude Ignored in lead V1 unless with abnormalities in other precordial leads Ignored in lead III unless with abnormalities in leads II and aVF C. Classification as to Timing of Myocardial Infarction CLASSIFICATION (A) Normal (B) Acute MI (C) Recent MI (D) Old MI TIMING Minutes to hours Hours to days Days to months ECG FINDINGS ST elevation + peaked or inverted T-waves + Q waves Q-waves +ST elevation + T-wave inversion Q-waves + Isoelectric ST-segment + T-wave inversion D. Posterior LV wall involvement Posterior wall ischemia, which is usually associated with lateral or inferior involvement, may be indirectly recognized by reciprocal or “mirror-image” ST depressions in leads V1 to V3 The posterior LV wall electrical activity is not represented in a typical standard surface ECG The anteroseptal leads (V1 to V3) are directed form the anterior precordium pointing towards the internal surface of the posterior myocardium E. Reciprocal Changes Pertains to ST-depression in leads opposite those demonstrating ST-elevation “Ischemia at a distance” Anterior MI: reciprocal change in inferior wall Inferior MI: reciprocal change in I, aVL, or anterior wall Lateral MI: reciprocal change in V1 or inferior wall 14 PULMONARY EMBOLISM (PE) McGinn-White sign: S1Q3T3 pattern (large S-wave in lead I, Q-wave in lead III, and inverted T-wave in lead III) indicating acute right heart strain Sinus tachycardia: most commonly cited abnormality T wave inversion on leads V1-V4: another most commonly cited abnormality (due to RV strain) Right bundle branch block Low amplitude deflections ELECTRICAL ALTERNANS Beat to beat variation in the QRS amplitude Seen in massive pericardial effusion and/or cardiac tamponade BUNDLE BRANCH BLOCKS V1 V6 Normal RBBB LBBB 15 A. Left Bundle Branch Block (LBBB) QRS duration >0.12 sec (complete LBBB) If <0.12 sec, then it is considered incomplete LBBB Broad, notched, or slurred R-waves in leads I, aVL, V5 and V6 Small or absent initial R-waves in right precordial leads (V1 and V2) followed by deep S-waves Absent septal Q-waves in leads I, V5 and V6 B. Right Bundle Branch Block (RBBB) QRS duration >0.12 sec (complete RBBB) If <0.12 sec, then it is considered incomplete RBBB Slurred S-wave in leads I and V6 RSR pattern in V1 (“bunny ears”) PERICARDITIS Acute Pericarditis A. Stages of Pericarditis Stage 1: Widespread ST elevation and PR depression with reciprocal changes in aVR (first two weeks) Stage 2: Normalization of ST changes; generalized T wave flattening (1 to 3 weeks) Stage 3: Flattened T waves become inverted (3 to several weeks) Stage 4: ECG returns to normal (several weeks onwards) 16 B. Pericarditis versus Myocardial Infarction PERICARDITIS Diffuse ST elevations which are concave upward MYOCARDIAL INFARCTION ST elevations which are convex upward ST elevation T-waves Q-waves Reciprocal Change PR depression T-wave usually not inverted unless ST is isoelectric Usually absent Unusual Usually present T-waves may begin to invert before ST becomes isoelectric Present Common Absent WOLFF PARKINSON WHITE (WPW) PATTERN Pre-excitation pattern: atrial impulse activates the ventricle earlier than would be expected if the impulse traveled by way of the normal AV conduction system o Triad of WPW: PR interval <120 msec, QRS >120 msec, (+) delta wave (slurred upstroke or initial portion of QRS complex) o Secondary ST-T wave abnormalities opposite that of the delta wave and QRS forces ARRHYTHMOGENIC RV DYSPLASIA (ARVD) Epsilon wave: a small positive deflection (‘blip’) buried at the end of the QRS complex, representing delay in depolarization of the right ventricular (RV) free wall and outflow tract Epsilon waves, found in 50% of patients with ARVD, are due to the slowed intraventricular conduction, hence the terminal notch in the last 1/3 segment of the QRS complex (which represents the right ventricular activation) BRUGADA ECG PATTERN Type 1 Type 2 Prominent coved ST-elevation displaying J-point amplitude or ST-elevation >2mm, followed by a negative T-wave >2 mm J-point elevation, >1 mm ST-elevation and a saddleback appearance, followed by a positive or biphasic T-wave Type 3 17 DEXTROCARDIA (“Right Sided Heart”) Absent R-wave progression in the chest leads (dominant S-waves throughout) Predominantly negative P-wave, QRS complex, and T-wave in lead I Low voltage in leads V3-V6 (because these leads are placed on the left side of the chest) Accidental reversal of the left and right arm electrodes may produce a similar ECG pattern in the limb leads but with normal QRS morphology in the precordial leads OTHER ECG FINDINGS Non-specific ST-T wave changes Poor R wave progression Low voltage complexes Electrolyte abnormalities T-wave inversion, ST segment depression/elevation not fulfilling the criteria for ischemia or infarction (as outlined above): flattened or slightly inverted T-waves, ST segments slightly above or below the isoelectric line R-wave in leads V1-V3 is < 3 small boxes Normal R-wave in V4-V6 QRS complexes <5 small boxes in limb leads or < 10 small boxes in chest leads Example: COPD, anasarca, obesity, myocarditis, moderate-sized to massive pericardial effusions Hypokalemia Prominent U wave + flattened T wave Hyperkalemia Peaked T-waves > 10 mm, wide QRS, sine wave pattern Hypocalcemia Prolonged QT interval Hypercalcemia Shortened QT interval CHEST RADIOGRAPH INTERPRETATION I. BASIC STEPS IN READING CHEST X-RAYS (CXR) Step 1: Identify general data Step 2: Determine view (PA, AP, lateral, decubitus) Step 3: Assess quality of film Step 4: Assess anatomy and determine abnormalities 18 STEP 1: IDENTIFYING GENERAL DATA OF THE PATIENT Patient name Date/Time CXR was taken Diagnosis of patient Indication for CXR STEP 2: DETERMINING THE VIEW Postero-Anterior View (PA) Scapula winged out, ribs and clavicles more angulated Arms at an angle with the body, with hands at waist Mongolian hat sign appreciated (formed by the C7 and T1 spinous + transverse processes) Heart not magnified Antero-Posterior View (AP) Scapula not winged out; clavicles more horizontal Arms parallel to body Mongolian hat sign not appreciated Heart and other structures magnified STEP 3: ASSESSING THE QUALITY OF THE FILM Inclusion Inspiratory Effort Exposure Obliquity Apices of the lungs to the costophrenic angles should be adequately visualized One should count >8 intercostal spaces, 6-8 anterior ribs, 9-11 posterior ribs Upper four thoracic vertebrae should be visualized Medial ends of both clavicles equidistant from midline The spinous process of the thoracic vertebra should be in the midline STEP 4: ASSESSING ANATOMY AND ABNORMALITIES A. General Structure Soft tissues and bones: soft tissue swellings, rib fractures, breast shadow, osteopenia/osteoporosis Trachea and mediastinum: carinal angle, tracheal position, mediastinal widening, masses Vessels: aortic knob and pulmonary arteries Diaphragm: right hemidiaphragm is usually higher than the left B. The Heart Assess CT ratio: >0.50 in PA view suggests cardiomegaly Cardiomegaly cannot be definitively ascertained on AP films, due to the possibility of magnification effects CHAMBER Left ventricular enlargement Right ventricular enlargement Left atrial enlargement Right atrial enlargement PA FILM Apex displaced inferiorly and laterally (drooping apex) Apex displaced superiorly and laterally (uplifted apex) Prominence of left atrial appendage Loss of cardiac waistline Widening of carinal angle (>70o) Double density sign on right cardiac border Bulging right heart border (height >1/2 of right cardiac silhouette and width 1/3 of right hemithorax) LATERAL FILM Obliteration of retrocardiac space Obliteration of retrosternal space Posterior displacement of the left mainstem bronchus on lateral film N/A C. The Lungs CP angle: blunting suggests minimal pleural effusion Pleura: check for pneumothorax, lesions Parenchyma: check for opacities, densities, infiltrates Lobes of the lungs: 19 o o Right Lung (3 lobes): Right upper lobe (RUL) + right middle lobe (RML) + right lower lobe (RLL) Left lung (2 lobes): Left upper lobe (LUL) + lingula + left lower lobe (LLL) II. COMMON CHEST X-RAY PATHOLOGIES Aortic Aneurysm Atelectasis Bronchiectasis Consolidation COPD/Emphysema Fibrosis Fungus Ball Hamartoma Pericardial Effusion Pleural Effusion Pneumomediastinum Pneumoperitoneum Pneumothorax Pulmonary edema Pulmonary Metastasis Mediastinum >30% of thoracic diameter, or mediastinum >8-10 cm Density in the area of the collapsed lung Displacement of interlobular fissures (direct sign) Surrounding structures deviated to the side of the collapsed lung (ipsilateral mediastinal shift) Crowding of vessels/bronchi Ipsilateral diaphragmatic elevation Appears as “bunches of grapes” (ring shadows) Tram-track lines Inhomogenous opacities Prominent air bronchogram sign Hyperaerated lungs Flattened hemidiaphragms Tubular heart Occasionally, bullae Decreased lung volume Shift of mediastinum and surrounding structures towards fibrotic area Blurred heart border or diaphragm with indistinct vascular markings in the areas of fibrosis Homogenous round opacity with a crescent sign Popcorn ball lesion Generalized enlargement of the cardiac shadow (“water bottle sign”) with normal vascular markings Blunting of costophrenic angles Meniscus sign Presence of gas between the mediastinal structures Presence of gas underneath the diaphragm Hyperlucent pulmonary area Loss of vascular markings beyond the visceral pleural line Mediastinal structures deviated to contralateral side (tension pneumothorax) Prominent hilar vessels (hilar fullness) in a bat-wing distribution Cephalization of vessels Kerley B lines Cannon ball lesions ARTERIAL BLOOD GAS (ABG) INTERPRETATION I. BASIC STEPS IN ABG INTERPRETATION Step 1: Determine the primary acid-base disorder and whether compensation is appropriate Step 2: Check for secondary acid-base disorders Step 3: Compute for anion gap and / when needed Step 4: Check oxygenation status 20 CHAPTER 2 CARDIOLOGY I. Introduction to Cardiology II. Approach to Patients with Cardiovascular Conditions 1. 2. 3. Cardiovascular History Cardiovascular Physical Examination The Cardiac Diagnosis III. Common Conditions in Cardiology 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Atherosclerosis and Dyslipidemia Hypertension Heart failure Chronic Stable Angina Pectoris Acute Coronary Syndrome A. Non-ST Elevation Acute Coronary Syndrome B. ST-elevation Myocardial Infarction Rheumatic Fever Valvular Heart Disease Pericarditis Cardiac tamponade Cardiomyopathy Atrial Fibrillation Peripheral Artery Disease CorPulmonale 37 SECTION 1 INTRODUCTION TO CARDIOLOGY CARDIOLOGY FORMULAS SV = EDV – ESV EF = SV EDV CO = HR X SV STROKE VOLUME (SV) Volume of blood pumped with each heart beat Normal range is 55-100mL (for a 70kg man, normal is ~70mL) SV = stroke volume (mL/beat) EDV = end diastolic volume (N: 65-240 mL) ESV = end systolic volume (N: 16-143 mL) EJECTION FRACTION (EF) Most useful index of LV function Fraction of blood ejected by the LV during systole EF = ejection fraction CARDIAC OUTPUT (CO) Volume of blood being pumped by the heart per minute Normal CO = 5.0 – 5.5 L/min CO = cardiac output (L/min) HR = heart rate (bpm) BLOOD PRESSURE (BP) SVR = systematic vascular resistance (resistance to blood flow through vascular system) BP = CO x SVR BODY SURFACE AREA (BSA) BSA = Weight (kg) x Height (cm) 3600 Better indicator of metabolic mass than body weight BSA = body surface area (m 2) CARDIAC INDEX (CI) CI = CO BSA SV = EDV – ESV Marker of cardiac function in relation to BSA, thus relating heart performance to the size of the individual Normal CI = 2.6 – 4.2 L/min/m2 If CI < 1.8 L/min/m2 suspect cardiogenic shock MEAN ARTERIAL PRESSURE (MAP) Defined as the average arterial pressure during a single cardiac cycle SBP = systolic blood pressure in mmHg DBP = diastolic blood pressure in mmHg Normal MAP = > 60mmHg can sustain organ perfusion (normally 70-110 mmHg) 38 PULSE PRESSURE (PP) Represents the force that the heart generates each time it contracts PP = SP – DP PP = pulse pressure: usually about 30-40 mmHg SP = systolic pressure (mmHg) DP = diastolic pressure (mmHg) Normal PP: if PP < 25% of the systolic value (eg, Low stroke volume, blood loss, aortic stenosis, tamponade) Wide PP: may reach up to 100mmHg (eg., exercise, atherosclerosis, aortic regurgitation, AV malformation, hyperthyroidism, aortic dilatation / aneurysm, fever, anemia, pregnancy, anxiety, beri-beri) Maximum HR = 220 – Age Target HR = Maximum HR x MAXIMUM HEART RATE Highest HR an individual can achieve without severe problems through exercise-induced stress TARGET HEART RATE A specific age-based pulse rate to be maintained 0.85 during aerobic exercise to ensure optimal cardiovascular function 39 SECTION 2 APPROACH TO PATIENTS WITH CARDIOVASCULAR CONDITIONS CARDIOVASCULAR HISTORY I. CARDINAL SYMPTOMS OF CARDIOVASCULAR DISEASE Chest pain or discomfort Dyspnea, orthopnea, paroxysmal nocturnal dyspnea (PND), wheezing Palpitations, dizziness, syncope Cough, hemoptysis Fatigue, weakness Pain in extremities with exertion (claudication) II. COMMON CAUSES OF CHEST PAIN CONDITION Cardiac Causes DURATION QUALITY Angina 2-10 mins Pressure, tightness, squeezing, heaviness, burning Unstable Angina 10-20 mins Similar to angina but often more severe Similar to angina Acute MI > 30 mins Similar to angina but often more severe Similar to angina Variable As described for angina As described for angina Aortic Stenosis Pericarditis LOCATION Retrosternal Radiation to neck, jaw, shoulders or arms (left) Retrosternal or toward apex Left shoulder and trapezius radiation Hours to days Sharp Sudden onset of unrelenting pain Tearing or ripping sensation; knife-like Sudden onset Pressure Variable Pressure Substernal Variable Pleuritic Unilateral, often localized Sudden onset; several hours Pleuritic Unilateral on side of ASSOCIATED FEATURES Precipitated by exertion, exposure to cold, psychologic stress Similar to angina but occurs with low levels of exertion or at rest Unrelieved by nitroglycerin May be associated with evidence of heart failure or arrhythmia Late-peaking systolic ejection murmur radiating to carotids Relieved by sitting up and leaning forward Pericardial friction rub is present Vascular Causes Acute Aortic Syndrome (Dissection) Pulmonary Embolism Pulmonary Hypertension Anterior chest, often radiating to the back, between shoulder blades Often unilateral, on the side of embolism Associated with hypertension and/or underlying connective tissue disorder (e.g., Marfan syndrome) Dyspnea, tachypnea, tachycardia, hypotension Dyspnea Signs of increased venous pressure Pulmonary Causes Pneumonia Pleuritis Spontaneous Pneumothorax or Dyspnea, cough, fever, rales, occasional pleural friction rub Dyspnea Decreased breath 40 pneumothorax sounds ipsilaterally Non-Cardiopulmonary Causes Esophageal Reflux 10-60 mins Burning Substernal, epigastric Esophageal Spasm Peptic Ulcer 2-40 mins Retrosternal Prolonged Pressure, tightness, burning Burning Gallbladder Disease Prolonged Aching or colicky Costochondritis Variable Aching Herpes Zoster Variable Sharp or burning Emotional/ Psychiatric Variable; may be fleeting Variable Epigastric. Substernal Epigastric, RUQ, substernal Sternal Dermatomal distribution Variable; may be retrosternal Worsened by postprandial recumbency Relieved by antacids Can closely mimic angina Relieved by food intake or antacids May follow meals Precipitated by fatty meals May be reproduced by localized or pinpoint pressure on exam Vesicular rash Situational factors precipitates symptoms Often with history of anxiety/depression CARDIOVASCULAR PHYSICAL EXAMINATION I. COMMON FINDINGS ON INSPECTION FINDING Central Cyanosis Peripheral Cyanosis Differential Cyanosis Homan’s Sign Kussmaul’s Sign Abdominojugular Reflux REMARKS Cyanosis due to right-to-left shunting, allowing deoxygenated blood to reach systemic circulation Cyanosis due to reduced extremity blood flow due to small vessel vasoconstriction Isolated cyanosis affecting the lower extremities but not the upper (seen in large PDA) Posterior calf pain on active dorsiflexion of the foot against resistance (suggestive of DVT) Rise or a lack of JVP with inspiration, associated with constrictive pericarditis Normally, the venous pressure should fall by at least 3 mmHg with inspiration Pressure over the upper abdomen (RUQ) for at least 10 seconds Positive response: sustained rise of >3 cm in JVP for at least 15 seconds after release of the hand II. COMMON FINDINGS ON PALPATION FINDING Cardiac Findings Normal LV Apex LV Enlargement RV Enlargement Peripheral Pulses PulsusParvus et Tardus Bifid Pulse PulsusParadoxus REMARKS Located in the left 5th ICS, mid-clavicular line Normal apex is a localized systolic outward thrust, less than 2.5 cm in diameter Apical impulse is shifted laterally & downwards Sustained systolic lift / heave in the left parasternal area A weak and delayed pulse that suggest severe aortic stenosis Two systolic peaks can be appreciated, described in hypertrophic obstructive cardiomyopathy (HOCM), with inscription of percussion and tidal waves Refers to a fall in SBP > 10mmHg with inspiration, seen in patients with: 41 PulsusAlternans pericardial tamponade, massive pulmonary embolism, hemorrhagic shock, severe obstructive lung disease, tension pneumothorax) Beat-to-beat variability of pulse amplitude seen in severe LV systolic heart failure III. COMMON FINDINGS ON AUSCULTATION A. Heart Sounds HEART SOUND First Heart Sound (S1) Second Heart Sound (S2) Third Heart Sound (S3) Fourth Heart Sound (S4) REMARKS Coincides with closure of the mitral valve and tricuspid valve (Systolic Pressure) Caused by the closure of the aortic and pulmonic valves (Diastolic Pressure) Indicates end of systole (or beginning of diastole) Best heard at the base; splitting is normally heard Variations include: - Widened Interval: RBBB, severe MR - Narrowly Split or Singular S2: Pulmonary arterial HPN - Fixed Splitting: ASD secundum - Paradoxical Splitting: LBBB, RV apical pacing, severe AS, HOCM, MI Coincides with early diastole or rapid ventricular filling It is caused by the flow of blood during rapid ventricular filling Best heard after S2 Coincides with late diastole or atrial systole (atrial contraction/slow ventricular filling) Diminished ventricular compliance increases resistance to ventricular filling More common in chronic LCH or active MI B. Common Auscultatory Areas of the Heart AREA LOCATION Aortic Area From the 2nd to 3rd ICS at the right sternal border Pulmonic Area From the 2nd to 3rd ICS at the left sternal border Tricuspid Area From the 3rd to 5th ICS at the left sternal border Mitral Area Near the apex of the heart at the 5th ICS in the left mid-clavicular line C. Grading of Murmurs GRADE Grade 1 Grade 2 Grade 3 Grade 4 Grade 5 Grade 6 D. Common Murmurs POSSIBLE DIAGNOSIS Pulmonic / Aortic Stenosis Aortic Regurgitation Tricuspid or Mitral Regurgitation VOLUME Faint; needs concentration Faint but can be heard readily by an experience observer Moderately loud Loud Very loud; heard with stethoscope lightly pressed on the skin Exceptionally loud; heard with stethoscope slightly above the chest wall Tricuspid or Mitral Stenosis Mitral Valve Prolapse DESCRIPTION OF MURMUR Systolic ejection murmur Early diastolic murmur Holosystolic (pansystolic) murmur if chronic Early systolic murmur if acute Murmur of TR usually increases with inspiration (Carvallo sign) Late diastolic murmur / rumble Systolic click with mid-to-late systolic murmur E. Other Sounds SOUND Opening Snap (OS) Pericardial Knock Tumor Plop DESCRIPTION High-pitched and occurs after a very short interval following S2 From the 2nd to 3rd ICS at the left sternal border From the 3rd to 5th ICS at the left sternal border THRILL? No No No Yes Yes Yes 42 THE CARDIAC DIAGNOSIS I. COMMON DIAGNOSTIC TESTS IN CARDIOLOGY DIAGNOSTIC Chest Radiograph (CXR) 12 Lead ECG Holter Monitoring Stress Testing Electrophysiology Study (EPS) Transthoracic Echocardiography (TTE) Transesophageal Echocardiography (TEE) Cardiac Magnetic Resonance Imaging (MRI) Computed Tomography Angiography (CTA) Nuclear Imaging Intravascular Ultrasound Coronary Angiography Cardiac Catheterization II. MAKING A CARDIAC DIAGNOSIS COMPONENT 1. Underlying Etiology 2. Anatomic Abnormalities 3. Physiologic Disturbances 4. Functional Disability REMARKS Widely used to visualize the heart and lungs Graphic recording of electric potentials generated by the heart to detect arrhythmias, conduction disturbances and ischemia Continuous ECG rhythm pattern for 24 hours or more to document paroxysmal rhythm abnormalities Non-invasive tool to evaluate the cardiovascular system’s response to stress under controlled conditions: o Exercise stress test: least invasive, makes use of a treadmill o Pharmacologic stress test: drugs are used to induce stress (e.g., dobutamines) Electrophysiological test of the heart involving intracardiac catheters with electrodes probing the endocardium to test conduction pathways and electrical activity Uses ultrasound waves to visualize heart chambers and valves Using Doppler studies, it can visualize blood flow through the heart Echocardiography that uses a specialized probe with an ultrasound transducer introduced into the esophagus to more accurately visualize posterior structures Differentiates soft tissues better than CT and allows comprehensive exams for assessment of size, morphology, function and tissue characteristics Displays vessels in the body (e.g., coronaries, aorta) May also be used to detect calcium in the coronary arteries Uses radioisotopes (Technetium, Thallium) taken up by viable myocardial cells to assess myocardial perfusion, viability & function (ischemic myocardium takes up less isotope) Percutaneous procedure that uses catheters to visualize the lumen and the interior wall of blood vessels Determines the patency and configuration of the coronary artery lumen by injecting contrast material into the coronary arteries Uses invasive monitoring and blood sampling through a catheter inserted into the heart to determine function, pressures, oxygenation, flow and volume of blood within the chambers and great vessels IMPORTANT QUESTIONS Congenital, hypertensive, ischemic or inflammatory? Which chambers are involved? Are they hypertrophied, dilated or both? Which valves are affected? Are they regurgitant and/or stenotic? Is there pericardial involvement? Has there been a myocardial infarction? Is an arrhythmia present? Is there evidence of congestive heart failure or of myocardial ischemia? How strenuous is the physical activity required to elicit symptoms? Example: Ischemic Heart Disease, Chronic Stable Angina Pectoris, CCS 3 Congestive Heart Failure NYHA FC III, in Sinus Rhythm 43 SECTION 3 COMMON CONDITIONS IN CARDIOLOGY ATHEROSCLEROSIS AND DYSLIPIDEMIA I. THE LIPID PROFILE A. Total Cholesterol TC = HDL + Non HDL TC = HDL + [LDL + VLDL] TC = HDL + LDL + TG 5 TC = total cholesterol in mg/dL HDL = high density lipoprotein in mg/dL LDL = low density lipoprotein in mg/dL VLDL = very low density lipoprotein in mg/dL (estimated by dividing TG level by 5) TG = triglycerides in mg/dL B. Individual Components 1. High Density Lipoproteins (HDL) Removes cholesterol from peripheral tissues via the reverse cholesterol transport Low HDL values < 40 mg/dL increased risk for heart disease 2. Non-High Density Lipoproteins (Non-HDL) Includes low density lipoproteins (LDL) and very low density lipoproteins (VLDL) Lower non-HDL cholesterol is desirable and is associated with a lower risk of heart disease Value can be estimated from other lipid values when non-HDL level is not directly available: NonHDL = TC – HDL NonHDL = LDL + VLDL NonHDL = LDL + TG 5 If VLDL values are not given, it can be estimated by dividing triglyceride levels by 5 Non-HDL are ApoB-100 containing atherogenic lipoproteins C. The Lipoproteins Composed of varying proportions of cholesterol, triglycerides and phospholipids LDL and HDL carry most cholesterol LIPOPROTEIN REMARKS Delivers dietary triglyceride to peripheral tissues Chylomicron Delivers cholesterol to the liver in the form of chylomicron remnants Delivers hepatic triglycerides to peripheral tissues (TG > cholesterol VLDL esters) Secreted by the liver Formed in the degradation of VLDL IDL Delivers triglycerides and cholesterol to the liver, where they are degraded into LDL Delivers hepatic cholesterol to peripheral tissues LDL Formed by lipoprotein lipase modification of VLDL in the peripheral tissues HDL Mediates reverse cholesterol transport (from periphery back to the liver) II. THE ACC/AHA 2013 GUIDELINES ON THE TREATMENT OF BLOOD CHOLESTEROL Statin therapy is recommended for individuals at increased atherosclerotic cardiovascular disease (ASCVD) risk who are most likely to experience a net benefit (benefit>harm) There is insufficient evidence to support continued use of specific LDL-C and/or non-HDL-C treatment “targets” Appropriate intensity of statin therapy should be used to reduce risk in those most likely to benefit 44 Non-statin therapies (e.g., fibrates, niacin), whether alone or in addition to statins, provide little benefit in risk reduction A. Four Major Statin Benefits Groups (ASCVD risk reduction outweighs risk of adverse events) 1. Acute coronary syndrome or history of MI 1 Patients with clinical ASCVD 2. Stable or unstable angina 3. Coronary or other arterial revascularization 4. Stroke, TIA or PAD 2 Primary elevations of LDL-cholesterol > 190mg/dL 3 Patients 40-75 years + LDL 70-189 mg/dL + Diabetes (without clinical ASCVD) 4 Patients 40-75 years + LDL 70-189 mg/dL + Estimated 10-year ASCVD risk > 7.5% (without clinical ASCVD or diabetes) B. Pooled Cohort Equations for ASCVD Risk Reduction For the fourth group, risk prediction is done by using the pooled cohort equations for ASCVD risk prediction (developed by the Risk Assessment Work Group) Calculator is not appropriate for those with known ASCVD Calculator is available online at http://tools.cardiosource.org/ASCVD-Risk-Estimator/ Computes for hard ASCVD events, including: o o o Non-fatal MI Death due to coronary heart disease (CHD) Fatal or non-fatal stroke Data includes: o Age, sex and race o Total cholesterol and HDL levels o Systolic BP o Treatment for hypertension o Diabetes o Smoking history C. Major Recommendations for Statin Therapy for ASCVD Prevention Clinical ASCVD LDL > 190 mg/dL Age > 75 High Intensity Statin (Class I) Moderate Intensity Statin (Class II) Diabetes + 40-75 10 year ASCVD risk > 7.5% and Age 40-75 Moderate Intensity Statin if risk is 5 to <7.5% (Class IIa) High Intensity Statin if 10 year ASCVD Risk > 7.5% (Class IIa) * 45 D. Intensity of Statin Therapy HIGH-INTENSITY THERAPY Daily dose lowers LDL by > 50% MODERATE-INTENSITY THERAPY Daily dose lowers LDL by 30 to <50% Atorvastatin 10-20 mg Rosuvastatin 10 mg Atorvastatin 40-80 mg Simvastatin 20-40 mg Rosuvastatin 20 mg Pravastatin 40 mg Lovastatin 40 mg Fluvastatin XL 80 mg Pitavastatin 2-4 mg INDICATED FOR Primary Prevention For 40-75 yrs + LDL 70-189 For 40-75 yrs + LDL 70-189 mg/dL: mg/dL: With diabetes* , or With diabetes and > With 5 to <7.5% 10-year risk 7.5% 10-year risk* (without ASCVD or MI) > 7.5% 10-year ASCVD risk For LDL-C >190 mg/dL: (without ASCVD or DM); moderate-to-high intensity > 21 years + primary LDL-C > 190 mg/dL (risk Individuals in whom high-density statin estimation is not therapy would be recommended but required) have characteristics predisposing them to statin-associated adverse effects** Secondary Prevention < 75 years + clinical > 75 years or safety concerns + ASCVD clinical ASCVD LOW-INTENSITY THERAPY Daily dose lowers LDL by < 30% Simvastatin 10 mg Pravastatin 10-20 mg Lovastatin 20 mg Fluvastatin 20-40 mg Pitavastatin 1 mg Individualized (e.g., patients who recover from mid-to-moderate muscle symptoms with higher doses of statins) *For patients 40-75 years old with LDL 70-189 mg/dL and DM, moderate- (class I recommendation) or high-intensity (class Iia recommendation if risk for ASCVD is >7.5%) may be considered, depending on the risk-benefit ratio. **Multiple or serious comorbidities, including impaired renal or hepatic function; history of previous statin intolerance or muscle disorders; unexplained alanine aminotransferase (ALT) elevations > 3 times the upper limit of normal (ULN); patient characteristics or concomitant use of drugs affecting statin metabolism; age > 75 years E. Monitoring Fasting lipid profile within 4-12 weeks after initiation or dose adjustment and every 3-12 months thereafter Indicators of anticipated therapeutic response to the recommended intensity of statin therapy (focus is on the intensity of the statin therapy as an aid to monitoring): o High-intensity statin therapy: LDL-C reduction of > 50% from the untreated baseline. o Moderate-intensity statin therapy: LDL-C reduction of 30% to <50% from the untreated baseline. III. MANAGEMENT A. Non-Pharmacologic Management (ACC/AHA 2013 Guide for Lifestyle Management for Reducing CV Risk) 1. Diet High in fruits and vegetables, whole grains; low fat; limit sweets DASH diet (Dietary Approaches to Stop Hypertension): o Rich in fruits, vegetables, whole grains, and low-fat dairy foods o Meat, fish, poultry, nuts and beans o Limited in sugar-sweetened foods and beverages, red meat, and added fats 2. Physical Activity 3-4 sessions a week, lasting 40 minutes per session Moderate-to-vigorous intensity physical activity 46 B. Pharmacologic Management DRUGS Statins Ezetimibe Cholestyramine MECHANISM OF ACTION HMG-CoA reductase inhibitor Inhibits melavonate: cholesterol precursor Cholesterol absorption inhibitor Bile acid sequestrant Prevents intestinal reabsorption of bile acids, this forcing liver to use cholesterol to make more bile acids as replacement LABORATOTY FEATURES 1st line of treatment 20-60% LDL reduction Doubling of dose: 6% additional lowering of LDL 15-20% LDL reduction Modest LDL reduction Can increase triglycerides Fibrates (Gemfibrozil, Fenofibrate) Upregulates lipoprotein lipase which increases hydrolysis of VLDL and chylomicrons 35-50% fasting triglyceride reduction Niacin Enhances activity of lipoprotein lipase, leading to decreased VLDL and triglyceride levels Reduces hepatic VLDL secretion into circulation and increases HDL significantly Only agent proven to raise HDL levels Omega-3-Fatty Acids (“Fish Oil”) Probucol Unclear, but it is thought to decrease catabolism of chylomicrons and increase affinity of LDL uptake Increases rate of LDL catabolism with strong anti-oxidant properties ADVERSE EFFECTS Myositis / myopathy Reversible elevation of AST / ALT May increase AST / ALT Bad taste, GI discomfort Decreased absorption of fat-soluble vitamins Contraindicated if triglycerides >200 mg/dL (relative) or 500 mg/dL (absolute) Myositis (increased risk if with concomitant statin use) Increase in AST / ALT Most common: nausea Flushing Hyperuricemia Impaired glucose tolerance Primary use: lower triglyceride levels (by reducing triglyceride synthesis in the liver) Bad / fishy taste Dyspepsia Reduces LDL levels Can reduce HDL levels Can reduce HDL QT interval F. Potency Equivalence of Statins DOSE OF AGENTS (mg) PERCENT REDUCTION Rosuvastatin Atorvastatin Simvastatin Pravastatin Total Cholesterol LDL-C 10 20 22% 27% 10 20 40 27% 36% 5 20 40 32% 42% 10 40 80 37% 48% 20 80 42% 54% Stained doses on same rows are equipotent (e.g., Rosuvastatin 10 mg is equivalent to atorvastatin 40 mg and both reduce LDL by 48%) 47 HYPERTENSION I. DIAGNOSIS OF HYPERTENSION Two or more elevated readings on at least 2 clinic visits over a period of one to several weeks Definition – adults with: o SBP > 140 mmHg, or o DBP > 90 mmHg II. CLASSIFICATION OF HYPERTENSION A. Classification as to Etiology Primary / Essential (most common) Secondary Hypertension B. Clues for Suspecting Secondary Hypertension Age of onset <20 or >50 years No family history of HPN DBP >100-120 mmHg Sudden increase in BP in a patient with stable Stage I HPN Poor BP control, despite good compliance Systemic findings (e.g. weight loss/gain, potassium abnormalities) C. Classification as to Stages CLASSIFICATION Normal Pre-hypertension Stage 1 Hypertension Stage 2 Hypertension Isolated Systolic Hypertension D. Definition of Terms VARIATION White Coat Hypertension Resistant Hypertension Orthostatic Hypotension SYSTOLIC (mmHg) <120 120-139 140-159 > 160 > 140 DIASTOLIC (mmHg) and <80 or 80-89 or 90-99 or > 100 and <90 DESCRIPTION At least three separate clinic-based measurements >140/90 mmHg and at least two non-clinic-based measurements <140/90 mmHg in absence of any evident of target organ damage Defined as high BP uncontrolled with three drugs or controlled with at least four anti-hypertensive drugs (including a diuretic) Fall in SBP >20 mmHg or in DBP >10 mmHg in response to assumption of the upright posture from a supine position within 3 minutes III. THE EIGHT JOINT NATIONAL COMMITTEE (JNC-8): MANAGEMENT OF HYPERTENSION A. Simplified Algorithm Age > 60 Age < 60 Any Age (+) Diabetes (-) CKD Any Age (+/-) Diabetes (-) CKD BP Goal <150 / <90 BP Goal <140 / <90 BP Goal < 140 / <90 BP Goal < 140 / <90 Initial Drug Thiazide, or ACEI or ARB, or CCB Initial Drug ACEI or ARB 48 B. Summary of the JNC-8 Recommendations on Management of Hypertension RECOMMENDATION No. 1 PATIENT POPULATION Patients > 60 years old Patients > 18 years old with CKD INITIATE THERAPY WHEN SBP > 150 mmHg or DBP > 90 mmHg DBP > 90 mmHg SBP > 140 mmHg SBP > 140 mmHg or DBP > 90 mmHg GOAL BLOOD PRESSURE SBP < 150 mmHg and DBP < 90 mmHg DBP < 90 mmHg SBP < 140 mmHg SBP < 140 mmHg and DBP < 90 mmHg No. 2 No. 3 Patients < 60 years old No. 5 Patients > 18 years old with DM SBP > 140 mmHg or DBP > 90 mmHg SBP < 140 mmHg and DBP < 90 mmHg RECOMMENDATION No. 6 PATIENT POPULATION Non-black population (including those with DM) No. 4 No. 7 No. 8 No. 9 INITIAL ANTIHYPERTENSIVE AGENT OPTIONS Thiazide-type diuretic Calcium channel blocker (CCB) Angiotensin-converting enzyme inhibitor (ACEI) Angiotensin receptor blocker (ARB) Thiazide-type diuretic Calcium channel blocker (CCB) Angiotensin-converting enzyme inhibitor (ACEI) Angiotensin receptor blocker (ARB) General black population (including those with DM) Patients > 18 years old with CKD (regardless of race or DM status) The main objective of hypertension treatment is to attain and maintain goal BP: If goal BP is not reached within a month, increase the dose of the initial drug or add a second drug (thiazide-type diuretic, CCB, ACEI or ARB) If goal BP cannot be reached with 2 drugs, add and titrate a third drug from the list Do not use an ACEI and an ARB together in the same patient If goal BP cannot be reached using only the drugs in recommendation 6 because of a contraindication or the need to use more than 3 drugs to reach goal, other classes can be used IV. MANAGEMENT OF HYPERTENSION A. Screening for Hypertension: Us Preventive Services Task Force recommends screening for high blood pressure in adults > 18 years old Screening: o Every 2 years if BP < 120/80 (normal) o Yearly if BP 120-139 / 80-89 (pre-hypertensive) B. Non-Pharmacologic Management: Lifestyle Management for Hypertension ASPECT GOAL Weight Reduction Attain and maintain BMI < 25 kg/m 2 Dietary Salt Reduction < 6 g NaCl/day Adapt DASH-type Dietary Plan Diet rich in fruits, vegetables, and low-fat dairy products Reduced content of saturated and total fat Moderation of Alcohol For those who drink alcohol, consume: Consumption o < 2 drinks/day in men o <1 drink/day in women Physical Activity Regular aerobic activity (e.g., brisk walking for 30 mins/ day) C. Common Drugs for Hypertension CLASS Diuretics Thiazide diuretics MECHANISM OF ACTION Selectively of Na/Cl symporter which ADVERSE EFFECTS Sexual impotence Hypokalemia REPRESENTATIVE DRUGS WITH DOSE RANGE mg/day (doses per day) Hydrochlorothiazide 6.25-50 mg OD 49 acts on the distal convoluted tubules, leading to enhanced NaCl excretion Dyslipidemia Hyperuricemia Hyperglycemia Loop diuretics Acts on the thick ascending limb of the Loop of Henle Hypokalemia Metabolic alkalosis Ototoxicity Hypocalcemia hypomagnesemia Potassium-Saving diuretics Spironolactone antagonizes action of aldosterone Triamterene & amiloride inhibit NaK exchange mechanism Hyperkalemia Gynecomastia (only for spironolactone) Metolazone 2.5-5 mg OD Indapamide 1.5mg OD Furosemide 40-80 mg OD Bumetanide 0.5-2 mg OD Ethacrynic Acid 25100 mg BID Spironolactone 12.5-100 mg OD Amiloride 5-20 mg OD Eplerenone 25-100 mg OD-BID Triamterene 25-100 mg OD Beta Blockers (BB) Cardioselective BB (B1) Non-Selective BB (B1/B2) Vasodilating BB (A1/B) Selectively inhibits B1-Receptors (less pulmonary effects) Inhibits both B1 and B2 receptors Bronchospasm Bradycardia AV block Metabolic syndrome Glucose intolerance Sleep disturbance Depression Combined A1 & Badrenergic receptor blockade Nebivolol: additional nitric oxide potentiating effect Atenolol 25-100 mg OD Metoprolol 50-400 mg/day Metoprolol XL 50200 mg OD Bisoprolol 2.5-20 mg OD Esmolol IV Propanolol 40-180 mg BID-TID Others: Pindolol, Timolol, Nadolol Cardvedilol 6.25-25 mg BID Nebivolol 5-10 mg OD Others: Labetolol Calcium Channel Blockers (CCB) Dihydropyridine Blocks L-type calcium channels Vascular effect > AV-node effect Tachyarrhythmia Edema Headaches Blocks L-type calcium channels AV node effect > vascular effect AV block (2nd and 3rd degree) Non-Dihydropyridine Trifascicular block Severe LV dysfunction Drugs Acting on the Renin-Angiotensin-Aldosterone-System (RAAS) ACE-Inhibitors Inhibits ACE Result: angiotensinI is not converted to angiontensin-II Cough Angioedema Hyperkalemia Renal agenesis Amlodipine 2.5-10 mg OD Felodipine 2.5-20 mg/OD Nifedipine 30-120 mg/day Diltiazem 120-360 mg/day Verapamil 120-480 mg/day Captopril 25-150 mg BID-TID Enalapril 2.5-20 mg/day Lisinopril 5-20 mg/day Perindopril 2.5-10 50 Angiotensin Receptor Blockers Competitive antagonism angiotensin-II Directly rennin, enzyme RAAS Direct Renin Inhibitor with inhibits the first in the Hyperkalemia Renal agenesis Less cough and angioedema Angioedema Hyperkalemia Cough Hyperuricemia mg/day Ramipril 2.5-10 mg OD Candesartan 8-32 mg OD Irbesartan 150-300 mg OD Losartan 25-100 mg OD Olmesartan 5-40 mg OD Telmisartan 20-80 mg OD Valsartan 80-320 mg OD Aliskiren 75-300 mg OD Other Anti-Hypertensives Alpha Blockers Blocks the postsynaptic A1receptors found in capacitance & resistance vessels Central Sympatholytics Activation receptors CNS Direct Vasodilators Release of nitric oxide, leading to arterial vasodilation of in A2the Postural hypotension Reflex tachycardia Sedation Xerostomia Impotence CNS side effects Rebound HPN on withdrawal Reflex tachycardia Headache Hypotension Lupus-like syndrome (for hydralazine) Hypertrichosis (for minoxidil) Prazosin 1-5 mg/day Terazosin 1-5 mg/day Doxazosin 1-8 mg OD Clonidine 75-150 mcg BID-TID Methyldopa 250500 mg BID-TID Hyrdrazaline 25 mg TID Minoxidil 2.5-80 mg/day V. MANAGEMENT OF UNCONTROLLED HYPERTENSION A. Differentiation Between Types of Uncontrolled Hypertension Usual BP Actual Target Organ Damage Management Monitoring SEVERE HYPERTENSION 180-220 / 110-130 mmHg None (asymptomatic) Long-acting oral medication can simple be restarted (usually occurs in chronic hypertensives who stopped taking medication) Require outpatient follow-up within 24-72 hours HYPERTENSIVE URGENCY >220/130 mmHg None HYPERTENSIVE CRISIS > 220/130 mmHg Present (brain, heart, kidney, retina or vessels) Short-acting oral medications Immediate reduction of BP with intravenous medication Require outpatient follow-up within 24 to 72 hours Admit for monitoring (ICU) 51 B. Common Intravenous (IV) Drugs for Hypertensive Emergencies DRUG DOSE 5-15 mg/hr as continuous infusion Nicardipine Starting dose 5 mg/hr, increase q1530 mins by 2.5 mg until goal BP achieved Nitroglycerin 5-200 ug/min 5 ug/min increase q5 mins 0.3-10 ug/kg/min, increase by 0.5 Nitroprusside ug/kg/min q5 mins until goal BP achieved Esmolol 0.5-10 ug/kg/min as bolus 500-300 ug/kg/min as infusion Labetalol 0.25-0.5 mg/kg; 2-4 mg/min until goal BP is reached, thereafter 5-20 mg/hr CONTRAINDICATIONS & SIDE EFFECTS Liver failure Can cause headaches Liver/kidney failure Can cause cyanide toxicity 2nd or 3rd degree AV block, systolic heart failure, bradycardia, COPD 2nd or 3rd degree AV block, systolic heart failure, bradycardia, COPD C. Recommended Treatment of Hypertensive Emergencies Based on End-Organ Involvement TYPE OF EMERGENCY TIMELINE, TARGET BP THERAPY* HPN Crisis with Several hours Labetalol, Nitroprusside, Retinopathy or Acute Nicardipine Target MAP: decrease by 20% t0 25% Renal Insufficiency HPN Encephalopathy Immediate Labetalol, Nicardipine, Nitroprusside Target MAP: decrease by 20% to 25% Acute Aortic Dissection Immediate Nitroprusside + Metoprolol, Labetalol Target SBP < 110 mmHg Acute Pulmonary Edema Immediate Nitroprusside + Loop Diuretic, Nitroglycerin Target MAP: 60-100 mmHg Acute Coronary Syndrome 1 hour Nitroglycerin, Labetalol Target MAP: 60-100 mmHg Acute Ischemic Stroke and 1 hour Labetalol, Nicardipine, BP > 220/120 mmHg Nitroprusside Target MAP: decrease by 5% Cerebral Hemorrhage and 1 hour Labetalol, Nicardipine, SBP > 180 mmHg or MAP > Nitroprusside Target SBP: < 180 mmHg and MAP 130 130 mmHg mmHg Several hours Phentolamine (after Cocaine intoxication benzodiazepines), Target SBP: < 140 mmHg Nitroprusside Labetalol + MgSO4 and oral Severe preeclampsia/ Immediate anti-HPN, Nicardipine eclampsia Target BP: < 160/105 mmHg Emergency Delivery of Fetus *Those underlined: first-line therapy VI. HYPERTENSION AND PREGNANCY Four categories of hypertension in pregnancy: 1. Pre-eclampsia: severe progressive multisystem disorder diagnosed by hypertension accompanied by any one of the following: o Proteinuria o BP of 160/110 mmHg or higher despite bed rest o Thrombocytopenia o Impaired liver function o Progressive renal insufficiency o Pulmonary edema o New-onset cerebral or visual disturbance 2. Chronic hypertension: hypertension predating pregnancy 3. Chronic hypertension with superimposed preeclampsia 4. Gestational hypertension: BP elevation after 20 weeks gestation in the absence of the additional systemic features listed above 52 HEART FAILURE (HF) I. ETIOPATHOGENESIS A clinical syndrome consisting of a constellation of clinical symptoms (dyspnea & fatigue) and signs (edema and rales) that lead to frequent hospitalization, a poor quality of life, and a shortened life expectancy. Etiologies: o Coronary artery disease (CAD): most common cause of HF in industrialized countries (60-75%) o Hypertension: cause of HF in 75% of patients o Idiopathic cardiomyopathy: 20-30% of depressed EF HF o Pulmonary heart disease: cor pulmonale, pulmonary vascular disorders o High output states: thyrotoxicosis, nutritional disorders (beriberi), excessive blood flow requirements, chronic asthma II. CLASSIFICATION / STAGES OF HEART FAILURE (HF) A. Classification Based on Function / Ejection Fraction (EF) TYPE EJECTION DESCRIPTION FRACTION Progressive disorder initiated by an Systolic Heart Depressed HF index event (e.g., MI, volume Failure or HF with < 40% overload, chronic anemia) that leads reduced EF (HfrEF) to a decline in the pumping capacity of the heart Proposed mechanisms include Diastolic Heart diastolic dysfunction and extraFailure or HF with Preserved EF cardiac mechanisms such as preserved EF > 40-50% increased vascular stiffness and (HfpEF) impaired renal function (still undefined and evolving) Occur when the body’s requirements Normal at first, then for oxygen and nutrients are High-Output Heart may decrease over increased and the demand outstrips Failure time what the heart can provide COMMON EXAMPLES CAD (e.g., MI) Dilated cardiomyopathy Valvular heart disease Pathologic hypertrophy (HOCM, HPN) Aging, fibrosis Restrictive cardiomyopathy Thyrotoxicosis Beriberi Chronic anemia Systemic arteriovenous shunting B. American College of Cardiology / American Heart Association (ACC/AHA) Stages of Heart Failure STAGE DESCRIPTION EXAMPLES At high risk for HF but without structural heart Patients with HPN, CAD, DM or patients using A disease or HF symptoms cardiotoxins or with family history of cardiomyopathy Structural heart disease but without signs or Patients with previous MI, LV systolic dysfunction, or B symptoms of HF asymptomatic valvular disease Structural heart disease with previous or Patients with known structural heart disease with C current symptoms of HF shortness of breath, fatigue, reduced exercise tolerance Refractory HF requiring specialized Patient who have marked symptoms at rest despite interventions maximal therapy (e.g., patients with recurrent D hospitalizations or cannot be safely discharged without special interventions) C. New York Heart Association (NYHA) Functional Classification NYHA DESCRIPTION Symptoms occur with greater than ordinary I physical activity Symptoms occur with ordinary physical activity II III IV Symptoms occur with less than ordinary physical activity Symptoms may be present even at rest COMMENTS No limitation of physical activity Can climb > 2 flights of stairs with ease Slight limitation of physical activity Can climb 2 flights of stairs but with difficulty Marked limitation of physical activity Can climb <1 flight of stairs Unable to carry on activity without symptoms Dyspnea at rest 53 III. CLINICAL MANIFESTATIONS A. Symptoms Fatigue and Shortness of Breath Orthopnea/Nocturnal Cough Paroxysmal Nocturnal Dyspnea Cheyne-Stokes Respiration Others B. Signs General Appearance and Vital Signs Cardiovascular Pulmonary Abdomen Extremities Cardinal symptoms Due to pulmonary congestion juxtacapillary J-receptors are activated cardiac dyspnea Redistribution of fluid from splanchnic and lower extremity into the central circulation on recumbency Severe dyspnea that awakens patient from sleep 1-3 hours after patient retires Increased pressure in the bronchial arteries In 40% of advanced HF: series of apnea hyperventilation hypocapnia Diminished sensitivity of the respiratory center to arterial PCO2 GI: anorexia, nausea, early satiety, abdominal fullness which may be due to congested liver and/or bowels CNS: confusion, disorientation, sleep and mood disturbance may be due to reduced cerebral perfusion Uncomfortable when lying flat, labored breathing Normal or low BP Cardiac cachexia Although essential, frequently does not provide information on the severity of HF JVP may be > 8 cm H2O Sinus tachycardia due to increased adrenergic activity Point of maximal impulse displaced due to cardiomegaly S3 (protodiastolic gallop) at the apex: usually in volume overloaded patients S4: usually in diastolic dysfunction Crackles: transudation of fluid from intravascular space to alveoli Expiratory wheezes: cardiac wheezing caused by peribronchial cuffing from congestion Pleural effusions: often bilateral; if unilateral, more often on the right Hepatomegaly with pulsation (if with significant TR) Ascites: increased pressure in the hepatic veins Jaundice: impairment of hepatic function due to congestion Peripheral edema: ankles and pre-tibial region Indurated and pigmented skin: long standing edema Peripheral vasoconstriction: cool extremities IV. DIAGNOSIS OF HEART FAILURE The diagnosis of HF is straightforward when the patients presents with classic signs and symptoms Key to diagnosis is a high index of suspicion A. Framingham Criteria for Heart Failure MAJOR CRITERIA MINOR CRITERIA Paroxysmal nocturnal dyspnea (PND) or orthopnea Ankle edema Neck vein distention Night cough Rales Dyspnea on exertion Cardiomegaly Hepatomegaly Acute pulmonary edema Pleural effusion S3 gallop Vital capacity decreased by 1/3 from maximal capacity Increased venous pressure > 16 cm H2O Tachycardia > 120 bpm Hepatojugular reflux Major or Minor Criteria: Weight loss > 4.5 kg in 5 days in response to treatment The diagnosis of HF requires simultaneous presence of at least: 1 Major Criteria, or 1 Major Criterion + 2 Minor Criterion 54 (use of minor criteria acceptable only if they cannot be attributed to another medical condition, such as pulmonary HPN, chronic lung disease, cirrhosis, ascites, nephrotic syndrome) B. Diagnostics in HF DIAGNOSTICS 2D Echocardiography with Doppler 12-L ECG Chest Radiography Cardiac Biomarkers (BNP) Complete Blood Count Serum Electrolytes, BUN, Crea, AST, ALT FBS, OGTT Lipid Profile FT4, TSH DESCRIPTION Most useful test, evaluation of ejection fraction (EF) Semi-quantitative assessment of LV size, function, wall motion abnormalities, valvular defects Assess cardiac rhythm, LV hypertrophy, prior MI A normal ECG virtually excludes LV systolic dysfunction Assess the cardiac size and shape and state of pulmonary vasculature Identify non-cardiac causes of symptoms Relatively sensitive markers for the presence of HF Increase with age and renal impairment Look for anemia, signs of infection, and bleeding (may precipitate / worsen HF) Assess for electrolyte disturbances, beginning cardiorenal syndrome, ischemic hepatitis or chronic passive congestion of the liver Assess for diabetes Assess for dyslipidemia Assess for thyroid hormone abnormalities V. MANAGEMENT OF HEART FAILURE A. Non-Pharmacologic Management and Basic Principles Sodium restriction: limit Na+ intake to 2-3 g/day in all patients with HF; and to less than 2 g/day in patients with moderate to severe HF Fluid restriction: generally unnecessary unless with hyponatremia (< 130 mEq/L) and volume overload Caloric supplement: for those with cardiac cachexia B. Pharmacologic Management for Prevention and Treatment of Chronic Heart Failure DRUG CLASS DESCRIPTION / MECHANISM DOSE Cornerstone of modern HF treatment Captopril 25-50 mg TID Interferes with RAAS by inhibiting the conversion of angiotensin I to Enalapril 2.5-10 mg BID ACE-Inhibitors angiotensin II Ramipril 2.5-10 mg OD Inhibits kininase which may lead to Lisinopril 5-20 mg OD increase in bradykinin (ACE-I induced cough) Angiotensin Receptor Use if ACEI intolerant (e.g., cough, Valsartan 40-160 mg BID Blockers angioedema) Candesartan 8-32 mg OD Losartan 25-50 mg OD Another cornerstone of modern HF treatment Carvedilol 3.125-25 mg BID Beta Blockers Interferes with sustained activation of Bisoprolol 1.25-10 mg OD the adrenergic nervous system, Metoprolol succinate 25-200 mg OD particularly the deleterious effects of B1 activation Inhibits action of aldosterone in Aldosterone Antagonist collecting duct Spironolactone 25-50 mg OD May also be used for fluid retention Eplerenone 25-50 mg OD (diuretic) For symptomatic LV dysfunction + Digoxin atrial fibrillation Digoxin 0.125-0.375 mg OD Add-on to standard therapy Reduces HR by inhibition of the Ivabradine 5-7.5 mg BID Ivabradine “funny channel” (If) in the SA node Primarily used for symptomatic 55 stable angina May be used for HF with systolic dysfunction in patients with sinus rhythm and HR > 70 bpm C. Management of Fluid Retention in Chronic HF DRUG CLASS DESCRIPTION / MECHANISM Act on the loop of Henle by reversibly Loop Diuretics inhibiting the reabsorption of Na+, K+, Cl in the thick ascending limb Reduce the reabsorption of Na+ and Cl in Thiazide and Thiazidethe first half of the distal convoluted tubule Like-Diuretics Tend to lose their efficiency with moderate to severe renal insufficiency (Crea > 2.5 mg/dL) Interfere with action at the vasopressin Arginine Vasopressin receptors Antagonists Primarily used for treatment of hyponatremia by stimulating free-water excretion and improving plasma Na+concentration D. Indications for Use of Drugs in HF CLASS ASYMPTOMATIC LV DYSFUNCTION (NYHA I) ACEI/ARB Yes Diuretic No B-Blocker Yes, if Post-MI Aldosterone Yes, if Recent MI Antagonist Digoxin May be considered* DOSE Furosemide 20-40 mg OD-BID Bumetanide 0.5-1.0 mg ODBID Hydrochlorothiazide 25 ODBID Indapamide 2.5 mg OD Metolazone 2.5-5.0 mg OD Tolvaptan 15 mg OD Satavaptan 25 mg OD SYMPTOMATIC HF (NYHA II) yes Yes, if with fluid retention Yes Yes WORSENING HF (NYHA III-IV) Yes Yes Yes Yes END-STAGE HF (NYHA IV) Yes Yes Yes Yes May be considered* Yes Yes *Digoxin may be considered for patients with NYHA-I for rate control in AF or when improved from more severe HF and in sinus rhythm E. Devices Used in HF Cardiac resynchronization therapy (CRT) or biventricular pacing: device used to restore synchrony of the left ventricle in patients with HF and a widened QRS complex Implantable cardioverter-defibrillator (ICD): device to treat tachyarrhythmias for primary / secondary prophylaxis against sudden cardiac death VI. ACUTE DECOMPENSATED HEART FAILURE (ADHF) A. Distinctive Phenotypes ACUTE DECOMPENSATION Typical Pulmonary Edema Low Output Cardiogenic Shock PRESENTATION Normo-hypertensive Usually not volume overloaded Severe pulmonary congestion with hypoxia Hypoperfusion with end-organ dysfunction Low pulse pressure, cool extremities Cardiorenal syndrome, hepatic congestion Hypotension, low cardiac output, end-organ failure Extreme distress, pulmonary congestion, renal failure MANAGEMENT Vasodilators, diuretics Vasodilators, diuretics, opiates O2 non-invasive ventilation Inotropic therapy Vasodilators Hemodynamic monitoring Inotropic therapy Mechanical circulatory support 56 B. Parenteral Therapy for Acute Decompensated HF DRUG CLASS SAMPLE DRUGS Inotropic Therapy Dobutamine (2-20 mcg/kg/min) Others: Milrinone, Levosimendan Vasodilators Nitroglycerine (10-20 mcg/kg/min) Others: Nesiritide, Nitroprusside, Serelaxin Furosemide (20-240 mg/day) Diuretics Bumetamide (0.5-5 mg/day) Others: Torsemide, Metolazone, Chlorthalidone, Spironolactone, Acetazolamide CHRONIC STABLE ANGINA PECTORIS (CSAP) Patients with ischemic heart disease (IHD) fall into two large groups: Chronic artery disease (CAD) who commonly present with chronic stable angina pectoris (CSAP) Acute coronary syndromes (ACS), discussed in the next section, are composed of: o Non ST-segment elevation acute coronary syndrome (NSTE-ACS) o ST-segment elevation acute myocardial infarction (STEMI) I. ETIOPATHOGENESIS Inadequate supply of blood flow and oxygen to a portion of the myocardium causing inadequate perfusion of myocardium supplied by an involved artery Most common cause: atherosclerotic disease of an epicardial coronary artery Obesity, insulin resistance, and T2DM are increasing and powerful risk factors for IHD II. CLINICAL MANIFESTATIONS A. Angina Typical history involves a man >50 years old or woman >60 years old who complains of chest discomfort: o Described as heaviness, pressure, squeezing, smothering or choking o Crescendo-decrescendo in nature o Usually lasts 2-5 minutes o Associated with physical exertion or stress o Radiation to either or both shoulders/arms, but does not radiate to the trapezius muscles o Relieved within 5-10 minutes by rest and/or sublingual NTG o Levine’s sign: hand placed over sternum with a clenched fist to indicate discomfort B. Canadian Cardiovascular Society Classification of Angina CCS CLASS DESCRIPTION I Angina occurs with greater than ordinary physical activity II Angina occurs with ordinary physical activity III Angina occurs with less than ordinary physical activity IV Angina may be present even at rest III. DIAGNOSIS A. Non-Invasive Diagnostics DIAGNOSTIC TEST ECG Stress Testing 2D Echo EXPECTED FINDINGS May be normal at rest ST-segment and T-wave changes, LV hypertrophy, intraventricular conduction disturbance (which may be non-specific) Most widely used for both diagnosis of IHD and estimating prognosis Involves recording the 12-lead ECG before, during and after exercise Used to assess left ventricular function in patients with CSAP and patients with a history of a prior MI, pathologic Q waves or clinical evidence of CHF Assess for wall motion abnormalities, ejection fraction, presence of thrombus, etc. 57 B. Indications for Coronary Angiography Patients with CSAP who are severely symptomatic despite medical therapy and considered for revascularization Patients with troublesome symptoms that present diagnostic difficulties in whom there is a need to confirm or R/O the diagnosis of IHD Patients with known or possible CSAP who have survived cardiac arrest Patients with CSAP or evidence of ischemia on noninvasive testing with clinical or laboratory evidence of ventricular dysfunction Patients at high risk of sustaining coronary events on noninvasive testing, regardless of symptoms IV. MANAGEMENT OF CSAP A. Pharmacologic Treatment for Angina DRUG CLASS EXAMPLES Anti-Ischemic Drugs Nitrates B-Blockers (BB) Calcium Channel Blockers (CCB) MECHANISM OF ACTION COMMENTS None of the longacting nitrates are as effective as SL NTG for the acute relief of angina Used for symptomatic relief May be discontinued with the disappearance of chest pain Common side effect limiting their use: headache At least 8 hour nitrate-free interval is recommended to avoid nitrate tolerance Isosorbide Dinitrate (ISDN) 10-40 mg BIDTID Isosorbide Mononitrate (ISMN) 30-240 mg OD NTG 0.3-0.6 mg SL, as needed up to 3 doses, 5 mins apart NTG Transdermal Patch 0.2-0.8 mg/hr OD (remove at bedtime for 12-14 hrs) Systemic venodilation with reduction in LV end-diastolic volume and pressure, thereby reducing myocardial wall tension and O2 requirements Dilation of epicardial coronary vessels Increased blood flow in collateral vessels Metoprolol 50-100 mg BID-QID Metoprolol XL 50-200 mg OD Carvedilol 3.125-50 mg BID Atenolol 50-100 mg OD Bisoprolol 5-20 mg OD Reduced myocardial O2 demand by inhibiting increases in HR, arterial pressure and myocardial contractility caused by adrenergic activation Cornerstone therapy for angina Shown to improve life expectancy following acute MI Coronary vasodilators that produce variable and dose dependent reductions in myocardial O2 demand, contractility, and arterial pressure Indicated in patients with: o Inadequate responsiveness to the combination of BB and nitrates o Adverse reactions to BB o Angina history of asthma or COPD o Sick sinus syndrome or significant AV conduction disturbances o Prinzmetal’s angina o Symptomatic Non-dihydropyridines Verapamil 80-120 mg TID-QID Diltiazem 30-90 mg TID-QID Dihydropyridines Amlodipine 2.5-10 mg OD Felodpine 2.5-10 mg OD 58 peripheral arterial disease Other Pharmacologic Agents for Angina Inhibitor of the IF ion channel (principal determinant of Ivabradine the SA node) 2.5-7.5 mg BID Slows the heart rate through a mechanism that is not associated with negative inotropic effects Dilates peripheral and coronary resistance vessels via Nicorandil ATP-sensitive K+ channels 10-20 mg BID Possess a nitrate moiety that promotes venous and coronary dilation B. Other Drugs for Stable Angina Pectoris DRUG CLASS EXAMPLES Aspirin 75-162 mg OD Antiplatelets Clopidogrel 75 mg OD Statins Rosuvastatin 10-20 mg OD Atorvastatin 10-80 mg OD Simvastatin 10-40 mg OD C. Coronary Interventions INTERVENTION Percutaneous Coronary Intervention (PCI) Coronary Artery Grafting (CABG) Bypass MECHANISM OF ACTION Irreversible inhibitor of platelet cyclooxygenase activity, interfering with platelet activation Oral agent that blocks ADP receptor-mediated platelet aggregation Act as HMG-CoA reductase inhibitor Exhibit pleiotropic effects: plaque stabilization and antiinflammatory effects Only works in patients who are in sinus rhythm Has anti-anginal efficacy similar to BB, nitrates & CCBs COMMENTS Chronic administration has been shown to reduce coronary events May be substituted for aspirin in those with aspirin hypersensitivity or those who cannot tolerate aspirin Can lower LDL cholesterol (25-50%), raise HDL cholesterol and lower triglycerides High intensity statin therapy should be given for patients with established IHD who are less than 75 years old, in the absence of contraindications DESCRIPTION Balloon dilatation usually accompanied by coronary stenting Most common indication: persistent or symptom-limiting angina pectoris, despite medical therapy, accompanied by evidence of ischemia during a stress test Indicated for those with three-vessel CAD or two-vessel CAD with involvement of the left anterior descending artery (LAD) or stenosis of the left main coronary artery ACUTE CORONARY SYNDROMES (ACS) Operational term that refers to a spectrum of conditions compatible with acute myocardial ischemia and/or infarction due to an abrupt reduction in coronary blood flow Patients with ACS are composed of: o Non-ST segment elevation acute coronary syndrome (NSTE-ACS): Non-ST segment elevation myocardial infarction (NSTEMI) Unstable angina (UA) ST elevation acute myocardial infarction (STEMI) 59 I. UNIVERSAL DEFINITON OF MYOCARDIAL INFARCTION A. Criteria for Acute MI “Acute MI” should be used when there is evidence of myocardial necrosis in a clinical setting consistent with acute myocardial ischemia. Under these conditions, any of the following criteria meet the diagnosis for MI: Detection of a rise and/or fall in cardiac biomarkers (preferably cardiac troponins/cTn), with at least one value above the 99th percentile with at least one of the following: o Symptoms of ischemia o New or presumed new significant ST-segment and/or T wave changes or new LBBB o Development of pathologic Q waves on the ECG o Imaging evidence of new loss of viable myocardium or new wall motion abnormality o Identification of an intracoronary thrombus by angiography or autopsy Cardiac death with symptoms suggestive of myocardial ischemia & presumed new ischemic ECG changes or new LBBB (Type 3) PCI-related MI (Type 4a) Stent thrombosis associated with MI (Type 4b) CABG-related MI (Type 5) B. Criteria for Previous Myocardial Infarction (any of the following): Pathologic Q waves with or without symptoms in the absence of non-ischemic causes Imaging evidence of a region of loss of viable myocardium that is thinned and fails to contract in the absence of a non-ischemic cause Pathologic findings of previous MI II. UNIVERSAL CLASSIFICATION OF TYPES OF MYOCARDIAL INFARCTION TYPE OF MYOCARDIAL INFARCTION DESCRIPTION (MI) Related to atherosclerotic plaque rupture, ulceration, fissuring, erosion or Type Spontaneous MI dissection with resulting intraluminal thrombus in one or more of the 1 coronary arteries that leads to decreased myocardial blood flow or distal platelet emboli with ensuing myocyte necrosis A condition other than CAD contributes to an imbalance between MI secondary to myocardial oxygen supply and/or demand, e.g., coronary endothelial Type Ischemic Imbalance dysfunction, coronary artery spasm, coronary embolism, 2 tachyarrhythmias/bradyarrhythmias, anemia, respiratory failure, hypotension, and hypertension with or without LVH MI resulting in Death Cardiac death with symptoms suggestive of ischemia and presumed new Type when Biomarkers are ischemic changes (or new LBBB), but death occurring before blood 3 Unavailable samples could be obtained MI associated with PCI defined by elevation of cTn values to >5x the 99th percentile of the upper reference limit in those with normal baseline values or a rise in cTn values >20% if baseline values are elevated and are stable or falling; AND either: MI related to PCI Symptoms suggestive of myocardial ischemia Type New ischemic changes on the ECG or new LBBB 4a Angiographic loss of patency of a major coronary artery or a side branch or persistent slow flow or no flow or embolization Imaging demonstration of new loss of viable myocardium or new regional wall motion abnormality MI associated with stent thrombosis is detected by coronary angiography Type MI related to Stent or autopsy in the setting myocardial ischemia and with a rise and/or fall in 4b Thrombosis cardiac biomarkers with at least one value >99th percentile of the upper reference limit MI associated with CABG – defined by elevation of cardiac biomarker values >10x the 99th percentile of upper reference limit in patients with Type MI related to CABG normal baseline values; AND either: 5 New pathologic Q waves or new LBBB, or New graft or new native coronary artery occlusion on angiogram, 60 or Imaging evidence of new loss of viable myocardium or new regional wall motion abnormality NON-ST ELEVATION ACUTE CORONARY SYNDROME (NSTE-ACS) I. ETIOPATHOGENESIS Most commonly caused by an imbalance O2 supply and demand, resulting from a partially occluding thrombus forming on a disrupted atherothrombotic coronary plaque on eroded coronary artery endothelium A. Four Basic Pathophysiologic Processes: Most common cause: plaque rupture or erosion with superimposed non-occlusive thrombus Dynamic obstruction (e.g., coronary spasm as in Prinzmetal’s variant angina) Severe mechanical obstruction Increased myocardial O2 demand (e.g., tachycardia) and/or decreased supply (e.g. anemia) B. Definition of Terms TERM Unstable Angina (UA) NSTEMI Prinzmetal Variant Angina DEFINITION Angina or equivalent ischemic discomfort with at least one of the following: Occurs at rest (or with minimal exertion), usually lasting >10 minutes Severe and of new onset (e.g., within the prior 4-6 weeks) of at least CCS III severity Occurs with crescendo pattern (e.g., distinctly more severe, prolonged or frequent than previous episodes) Clinical features of UA plus evidence of myocardial necrosis (elevated cardiac biomarkers) Ischemic pain that occurs at rest but not usually with exertion, associated with transient ST-segment elevation Due to transient, focal spasm of an epicardial coronary artery II. CLINICAL MANIFESTATIONS A. Typical Chest Pain Chest discomfort is typically severe and has at least one of the following features: o Occurs at rest (or with minimal exertion), lasting >10 minutes o Relatively recent onset (within prior 2 weeks) o Occurs with a crescendo pattern (e.g., more severe, prolonged or frequent) B. Symptoms & Signs of NSTE-ACS SYMPTOMS Chest pain radiating to the neck, left shoulder, and left arm Dyspnea Diaphoresis Anxiety, restlessness SIGNS Pale cool skin Sinus tachycardia S3 or S4 Basilar rales Hypotension III. DIAGNOSIS OF NSTE-ACS A. 12-Lead Echocardiogram (ECG) ST-segment depression, transient ST-segment elevation or T-wave inversion T-wave changes: sensitive for ischemia but less specific (unless new & deep T-wave inversions > 0.3 mV) If initial ECG is not diagnostic – serial ECGs should be done to detect ischemic changes if patient remains symptomatic with a high suspicion for ACS B. Cardiac Biomarkers Elevated levels distinguish patterns with NSTE-ACS from UA 61 Serial cardiac troponin I or T levels should be obtained at presentation and 3-6 hours after symptom onset to identify a rising and/or falling pattern of values Additional levels should be obtained beyond 6 hours in patients with normal levels on serial examination when ECG and/or clinical presentation confer an intermediate or high index of suspicion for ACS 1. Advantages and Disadvantages of the Common Cardiac Biomarkers CARDIAC TROPONINS Powerful tool for risk stratification Greater sensitivity and specificity than Advantage CK Detection of recent MI up to 2 weeks after onset (remain elevated 7-10 days after MI) Low sensitivity in very early phase of MI (<6 hours after symptom onset) and requires repeat measurement at 8-12hours if negative Disadvantage Limited ability to detect late minor reinfarction Minor troponin elevations can be caused by azotemiz/CKD, CHF, myocarditis or pulmonary embolism 2. Timing of Cardiac Markers CARDIAC BIOMARKER Troponin-T Troponin-I CK-MB TIME TO DETECTION 3-12 hrs 3-12 hrs 4-8 hrs PEAK 24 hours 24 hours 24 hours CK-MB Rapid, cost-efficient, accurate assays Ability to detect early reinfarction Loss of specificity in setting of skeletal muscle disease of injury, including surgery and IM injections With contemporary troponin assays, CKMB is not useful for diagnosis of ACS DURATION 5-14 days 5-10 days 2-3 days C. Risk Stratification: TIMI SCORE for NSTE-ACS Prognostication scheme, which categorizes patients based on risk of all-cause mortality, new or recurrent MI, or severe ischemia requiring urgent revascularization COMPONENTS Age > 65 years > 3 CAD factors Known CAD (> 50% stenosis) Aspirin use in the [ast 7 days Severe angina in last 24 hours Elevated cardiac markers ST deviation > 0.5mm POINTS 1 point 1 point 1 point 1 point 1 point 1 point 1 point INTERPRETATION Risk Total Score: 0-7 points High Risk Score: > 3 points (13% mortality) IV. MANAGEMENT OF NSTE-ACS A. Standard and Anti-Ischemic Therapy THERAPY Non-Pharmacologic Nitrates DESCRIPTION Bed rest with continuous ECG monitoring Supplemental oxygen if O2 sat <94% Avoid in: SL nitrate (ISDN 5 mg/tab) q5 SBP <90 mmHg or >30 mmHg mins x total of 3 doses below baseline IV NTG in first 48 hours (5-10 Severe bradychardia <50 bpm mcg/kg/min, max 200 Tachycardia >100 bpm in mcg/kg/min) for persistent absence of symptomatic HF ischemia, HF or HPN Suspected RV infarction Decrease in angina symptoms Those who received sildenafil for the past 24 hours (may potentiate hypotension) 62 Beta-Blockers (BB) Nondihydropyridine Calcium Channel Blockers ACE Inhibitors Not given in patients with: Signs of acute HF Low output states (SBP <90, Started within 24 hours HR <50) Metoprolol succinate, PR interval > 0.24 secs, 2nd or carvedilol or bisoprolol 3rd degree AVB without a pacemaker Active asthma or reactive airway disease Recommended for recurrent ischemia after appropriate use of BB and nitrates For those with contraindications to beta-blockers Verapamil or diltiazem Given orally within 24 hours in patients with congestion and/or LVEF < 40% ARBs may be given if patient intolerant to ACE inhibitors Captopril 6.25-12.5 mg PO q8 B. Anti-Platelet Therapy Initial treatment should begin with aspirin In the absence of a high risk of bleeding, patients with NSTE-ACS should also receive a P2Y12 inhibitor for up to 12 months (at least 12 months if patient is to undergo PCI with stenting): clopidgrel, ticagrelor, prasugrel THERAPY DESCRIPTION Platelet cyclooxygenase inhibitor Aspirin Dose: 165-325 mg loading dose, then 80-162 mg OD maintenance dose indefinitely Thienopyridine Clopidogrel Inactive prodrug that is converted into an active metabolite that causes irreversible blockade of the platelet ADP P2Y12inhibitor Dose: 300-600 mg loading dose, then 75 mg OD Non-thienopyridine Novel, potent, reversible platelet ADP P2Y12inhibitor Ticagrelor May be used in patients who are treated either by an invasive or conservative strategy Dose: 180 mg loading dose, then 90 mg BID Thienopyridine Also a platelet ADP P2Y12 antagonist, but achieves a more rapid onset and higher level of platelet inhibition than clopidogrel Approved for ACS patients following angiography in whom PCI is planned (it Prasugrel has not been found to be effective in patients treated by a conservative strategy) Contraindicated in patients with prior stroke / TIA or a high risk of bleeding Dose: 60 mg loading dose, then 10 mg OD (if patient is to undergo early invasive management) C. Anticoagulation Therapy THERAPY Unfractioned Heparin (UFH) Enoxaparin Fondaparinux DESCRIPTION Mainstay of therapy Target aPTT 50-70 seconds (ratio of 1.5-2.5) Dose: 60 U/kg IV bolus (maximum 4,000 U), then 12 U/kg infusion (1000 units/hour) for 48 hours or until PCI is performed (most trials continued therapy for 2-5 days) Superior to UFH in reducing recurrent cardiac events, especially in patients managed conservatively Dose: 30 mg IV loading dose, then 1 mg/kg SC q12 for the duration of hospitalization or until PCI is performed Indirect factor Xa inhibitor 63 Equivalent in efficacy to enoxaparin, but with lower risk of major bleeding Dose: 2.5 mg SC OD for duration of hospitalization or until PCI is performed D. Statins High intensity statin therapy should be initiated or continued Early administration of statins (e.g., Atorvastatin 80 mg/day) has been shown to reduce adverse outcomes E. Conservative versus Early Invasive Strategy Conservative strategy (low risk patients): anti-ischemic therapy and antithrombotic therapy followed by “watchful waiting” (close observation) Early-invasive strategy (for high risk patients): following treatment with anti-ischemic and antithrombotic agents, angiography is carried out within 48 hours, followed by coronary revascularization (PCI or CABG) CONSERVATIVE MEDICAL MANAGEMENT EARLY INVASIVE MANAGEMENT (Ischemia-Guided Strategy) (Revascularization) Low risk score (TIMI 0 or 1) Recurrent angina or ischemia at rest or with lowlevel activities despite intensive medical therapy Low risk troponin-negative female patients Elevated cardiac biomarkers (TnT or Tnl) Patient or physician preference in the absence of high-risk features New or presumably new ST segment depression CHF symptoms, rales, MR Reduced left ventricular function (LVEF < 40%) Sustained ventricular tachycardia PCI < 6 months or prior CABG High-risk findings from noninvasive testing Hemodynamic instability Mild-to-moderate renal dysfunction DM High TIMI risk score > 3 IV. PRINZMETAL’S VARIANT ANGINA Syndrome of severe ischemic pain that usually occurs at rest and associated with transient ST elevation Caused by focal spasm of an epicardial coronary artery (most commonly the right coronary artery) Diagnostic hallmark: coronary arteriography demonstrates transient coronary spasm Main therapeutic agents: nitrates and calcium channel blockers Aspirin may increase severity of ischemic episodes ST-ELEVATION MYOCARDIAL INFARCTION (STEMI) I. ETIOPATHOGENESIS Acute plaque rupture is central to the pathogenesis of STEMI Occurs when coronary blood blow decreases abruptly after a thrombotic occlusion of a coronary artery previously affected by atherosclerosis II. CLINICAL MANIFESTATIONS Diagnosed similarly as NSTE-ACS (e.g., clinical features, increased cardiac biomarkers) but with ECG findings evolving in a temporal pattern (see ECG Reading in Chapter 1) III. DIAGNOSIS AND RISK STRATIFICATION FOR STEMI A. Killip Scoring for STEMI CLASS DESCRIPTION No rales or signs of pulmonary or venous congestion Class Normal BP I Class II Moderate HF, bibasal rales Normal BP S3 gallop Tachypnea or signs of right-sided CHF (venous or hepatic congestion) Severe HF RISK OF MORTALITY 0-5% 10-20% 64 Class III Class IV (+) mid-basal rales and pulmonary edema (+) S3 and S4 Normal BP Shock with SBP <90 mmHg & evidence of peripheral vasoconstriction Peripheral cyanosis Mental confusion and oliguria 35-45% 85-95% B. TIMI Risk Score for STEMI TIMI risk score for STEMI: predicts 30-day mortality Designed for risk assessment early after patient presentation and thus does not incorporate noninvasive and invasive data COMPONENTS POINTS INTERPRETATION Historical Age 65-74 2 points Age > 75 3 points Risk Total Score: DM, Hypertension, Angina 1 point 0-14 points Examination SBP < 100 mmHg 3 points High Risk Score: HR > 100 bpm 2 points > 5 points Killip II-IV 2 points (12% mortality) Weight < 67 kg 1 point Presentation Anterior ST elevation or LBBB on ECG 1 point Time to Treatment > 4 hours 1 point IV. MANAGEMENT OF STEMI A. Pre-hospital Management of STEMI Major components: o Recognition of symptoms o Rapid deployment of an emergency medical team capable of performing resuscitative maneuvers o Expeditious transportation o Expeditious implementation of reperfusion therapy Most out-of-hospital deaths from STEMI are due to sudden ventricular fibrillation Majority of deaths occur within 24 hours of the onset of symptoms (over half occur in the 1st hour) B. Reperfusion Therapy: Primary Goal of Management Reperfusion Therapy (fibrinolysis or PCI) should be administered to all eligible patients with STEMI with symptom onset within the last 12 hours o Primary PCI: recommended method of reperfusion when it can be performed in a timely fashion o Fibrinolysis: administered at non-PCI-capable centers FIBRINOLYSIS / THROMBOLYSIS INVASIVE STRATEGY (PCI) Generally preferred if: Generally preferred if: Early presentation (< 3 hours of symptom onset) Available PCI laboratory with surgical backup o Medical contact-to-balloon or door-to-balloon Invasive strategy is not available: < 90 minutes Delay to invasive strategy: o Door-to-balloon minus door-to-needle < 1 hr o Prolonged transport High risk STEMI (cardiogenic shock, Killip > 3) o Door-to-balloon minus door-to-needle time >1 hr Contraindications to fibrinolysis o Medical contact-to-balloon or door-toLate presentation (symptom onset > 3 hours) balloon time >90 minutes Diagnosis of STEMI is in doubt Fibrinolytic agents: Percutaneous coronary intervention (PCI) or o Streptokinase percutaneous transluminal coronary angioplasty o Tissue plasminogen activators (PTCA): balloon angioplasty and stenting Adjunct anti-platelet therapy with fibrinolysis: Aspirin continued indefinitely Anti-platelet therapy during Primary PCI: 65 Clopidogrel for at least 14 days up to 1 year o o Aspirin indefinitely after PCI One P2Y12-receptor inhibitor continued for 1 year for those who receive a stent: Clopidogrel Prasugrel (not used if + prior stroke/TIA) Ticagrelor Adjunctive anticoagulant therapy with fibrinolysis: given for a minimum of 48 hours or until revascularization is performed (same dose as in NSTE-ACS) Anticoagulant therapy during primary PCI: o Unfractioned heparin (UFH) o UFH o Enoxaparin o Bivalirudin o Fondaparinux Fibrinolysis is still reasonable if symptom onset is within 12-24 hours as long as there is evidence of ongoing ischemia (although primary PCI is preferred for this population) CONTRAINDICATIONS TO FIBRINOLYSIS ABSOLUTE CONTRAINDICATIONS RELATIVE CONTRAINDICATIONS Previous intracranial hemorrhage History of chronic, severe, poorly controlled HPN Structural cerebral vascular lesion (e.g., Significant HPN at initial evaluation (SBP > 180 AVM) mmHg or DBP > 110 mmHg) Malignant intracranial neoplasm History of previous ischemia stroke > 3 months Ischemic stroke within 3 months except Dementia acute ischemic stroke within 4.5 hours Intracranial pathology not covered in absolute Suspected aortic dissection contraindications Active bleeding / bleeding diathesis (except Traumatic or prolonged (>10 minutes) CPR mense) Major surgery (<3 weeks) Closed-head or facial trauma within 3 months Recent (within 2-4 weeks) internal bleeding Intracranial/intraspinal surgery within 2 Noncompressible vascular punctures months Pregnancy Severe uncontrolled hypertension Active peptic ulcer (unresponsive to emergency therapy) Oral anticoagulant therapy For streptokinase, previous treatment within the previous 6 months AVM: Arteriovenous malformation CPR: cardiopulmonary resuscitation C. Other Routine Medications for STEMI THERAPY Beta Blockers RAAS Inhibitors Statins DESCRIPTION Should be initiated in the first 24 hours, except if with signs of HF, low output state, increased risk for cardiogenic shock, or other contraindications (PR interval > 0.24, 2nd or 3rd degree AVB, active asthma, reactive airway disease) ACE-inhibitors should be initiated in the first 24 hours to all patients with anterior wall STEMI, HF or EF < 40% ARB may be used for those intolerant to ACEinhibitors High intensity statin therapy should be initiated or continued D. Supportive Care THERAPY Activity DESCRIPTION First 12 hours: bed rest Next 12 hours: dangling of feet at bedside and sitting in a chair 2nd and 3rd day: ambulation in the room with increasing duration and frequency to a goal of 185 cm (600 ft) at least 3x a day 2 weeks: resumption of work and sexual activity 66 Nothing or only clear liquids (due to risk of emesis and aspiration) for the first 4-12 hours Use of stool softener Many require sedation during hospitalization to withstand period of enforced inactivity Diet Sedation E. Secondary Prevention and Long Term Management THERAPY DESCRIPTION Smoking Complete cessation BP Control BP <140/90 or <130/80 if CKD or DM Lipid Management High dose statins <7% of total calories as saturated fats and <200 mg/day total cholesterol Physical Activity 30 minutes of moderate intensity aerobic exercise, 3 to 4 days per week Weight BMI 18.5 – 24.9 kg/m2 Management Waist circumference: women <35 inches, men <40 inches DM Management HbA1c <7% Anti-platelets Aspirin or P2Y12-receptor inhibitors RAAS Blockers ACEI in stable high-risk patients (anterior MI, previous MI, Killip > II, EF <40%) Beta Blockers Continued indefinitely IV. USUAL COMPLICATIONS OF STEMI COMPLICATION FREQUENCY Ventricular 1-3% in those who Septal Rupture did not undergo (VSR) reperfusion Ventricular Free Wall Rupture Papillary Muscle Rupture 0.8-6.2% 1% (posteromedial more frequently affected than anterolateral muscle) DESCRIPTION Bimodal peak (within 24 hours & 3-5 days; can range from 1-14 days) Presents with chest pain, SOB and hypotension Holosystolic murmur, S3, accentuated 2 nd heart sound, pulmonary edema, RV and LV failure, cardiogenic shock Bimodal peal (within 24 hours & 3-5 days; can range from 1-14 days) Presents with angina, pleuritic or pericardial chest pain, syncope, hypotension, arrhythmia, nausea, restlessness, hypotension and sudden death JV distention (29%), pulsus paradoxus (47%), electromechanical dissociation and cardiogenic shock Bimodal peak (within 24 hours & 3-5 days; can range from 1-14 days) Abrupt onset of dyspnea, pulmonary edema, and hypotension Soft murmur in most cases, no thrill, variable signs of RV overload, severe pulmonary edema, cardiogenic shock Hypercontractile LV, torn papillary muscle or chordae tendinae, flail leaflet and severe MR on echo with color flow RHEUMATIC FEVER (RF) I. ETIOPATHOGENESIS Multi-system disease resulting from autoimmune reaction to infection with Group A Beta-Hemolytic Streptococcus In RF, antibodies against M-proteins of certain strains of Streptococcus cross-react with tissue glycoproteins in the heart, joints and other tissues (“molecular mimicry”) II. CLINICAL MANIFESTATIONS Latent period of 3 weeks (ranges from 1 to 5 weeks) between the precipitating infection and the appearance of the clinical features of ARF with the exception of chorea and indolent carditis which may follow prolonged talent period lasting up to 6 months Most common clinical presentation: polyarthritis and fever A. Major Manifestations Carditis (up to 60%) Pancarditis involving the pericardium, myocardium and endocardium Hallmark is valvular damage 67 Migratory Polyarthritis (75%) Sydenham’s Chorea (<10%) Erythema Marginatum Subcutaneous Nodules B. Minor Manifestations Clinical Laboratory findings Characteristic manifestation is mitral regurgitation Typically migratory over a period of hours Most often asymmetric and affecting large joints (ankles, wrists, knees, elbows) Highly responsive to salicylates and NSAIDs Involuntary jerking movements mostly affecting the head and upper limbs Commonly occurs in females and in the absence of other manifestations Usually resolves completely within 6 weeks Evanescent pink macular eruption with round borders and central clearing Usually concentrated on the trunk, sometimes on the limbs, but almost never on the face Painless small lumps found over extensor surfaces of joints Usually a delayed manifestation (2-3 weeks after onset) Commonly associated with carditis Arthralgia (joint pains), fever Elevated acute phase reactants (ESR/CRP), prolonged PR interval on ECG C. Supporting Evidence of a Preceding Streptococcal Infection within the last 45 days Elevated or rising anti-streptolysin-O or other streptococcal antibody, or A positive throat culture, or Rapid antigen test for group-A Streptococcus, or Recent scarlet fever III. DIAGNOSIS A. The Revised Jones Criteria: Diagnosis of Rheumatic Fever (RF) and Rheumatic Heart Disease (RHD) DIAGNOSTIC CATEGORIES CRITERIA Primary episode of RF Evidence of preceding group-A streptococcal infection; PLUS: 2 major criteria, or 1 major + 2 minor criteria Recurrent RF in a patient Evidence of preceding group-A streptococcal infection; PLUS: without established RHD 2 major criteria, or 1 major + 2 minor criteria Recurrent RF in a patient with Evidence of preceding group-A streptococcal infection; PLUS: established RHD 2 minor criteria Rheumatic chorea Other major manifestations or evidence of group-A streptococcal infection not Insidious onset rheumatic required carditis Chronic valve lesions of RHD (patients presenting for the 1st time with pure MS or mixed Do not require any other criteria to be diagnose as having RHD MV disease and/or AV disease) B. Criteria for Echocardiographic Diagnosis of Rheumatic Heart Disease (RHD) in Individuals <20 years of age Definite RHD (either A, B, C or D) A. Pathologic MR + > 2 morphologic features of RHD of the mitral valve (MV) B. MS mean gradient >4 mmHg C. Pathologic AR + > 2 morphologic features of RHD of the aortic valve (AV) D. Borderline disease of both the MV and AV Borderline RHD (either A, B, C) A. > 2 morphologic features of RHD of the MV without pathologic MR or MS B. Pathologic MR C. Pathologic AR Normal Echocardiographic Findings (all of A, B, C and D) A. MR that does not meet all four Doppler criteria (physiologic MR) B. AR that does not meet all four Doppler criteria (physiologic AR) 68 C. An isolated morphologic feature of RHD of the MV (e.g., valvular thickening), without any associated pathologic stenosis or regurgitation D. Morphologic feature of RHD of the AV (e.g., valvular thickening), without any associated pathologic stenosis or regurgitation Definitions of Pathologic Regurgitation & Morphologic Features of RHD Pathologic MR: all of the following – seen in 2 weeks; in at least 1 view, jet length 2 cm; peak velocity > 3 m/s; pansystolic jet in at least 1 envelope Pathologic AR: all of the following – seen in 2 views; in at least, jet length > 1 cm; peak velocity > 3 m/s’ pandiastolic jet in at least 1 envelope Morphologic features of RHD in MV: anterior MV leaflet thickening > 3 mm; chordal thickening; restricted leaflet motion; excessive leaflet tip motion during systole Morphologic features of RHD in AV: irregular of focal thickening; coaptation defect; restricted leaflet motion; prolapse IV. MANAGEMENT OF RHEUMATIC FEVER A. For Acute Management Penicillin: o PO: Pen V 500 mg BID or Amoxicillin 50 mg/kg daily x 10 days o IM: single dose of 1.2 M units Benzathine Penicillin G Aspirin 4-8 g/d in 4-5 divided doses up to 2 weeks May add prednisone 1-2 mg/kg/day up to 4 max of 3 weeks Carbamazepine or valproic acid For Infection For Arthritis / Mild Carditis For Moderate-Severe Carditis For Severe Chorea B. For Prophylaxis of Rheumatic Fever Primary prophylaxis for RH: to treat group-A streptococcal URTI and eradicate the organism to prevent an initial attack of acute RF Secondary prophylaxis for RF: to prevent colonization and/or infection in patients who had a previous attack of RF to prevent recurrence of RF 1. Drugs Available for Secondary Prophylaxis Benzathine Penicillin G 1.2 M units q 2-4 weeks (best) Penicillin VK 250 mg/cap BID Erythromycin 250 mg/cap BID (if allergic to Penicillin) 2. Duration of Secondary Prophylaxis CATEGORY RF without Carditis RF with Carditis, but no residual valvular disease RF with persistent valvular disease DURATION OF PROPHYLAXIS 5 years after last attack or until 21 y/o (whichever is longer) 10 years after last attack or until 21 y/o (whichever is longer) 10 years after last attack or until 40 y/o (sometimes lifetime) VALVULAR HEART DISEASE (VHD) I. STAGES OF PROGRESSION OF VHD CLASS STAGE A STAGE B (At Risk) (Progressive) General Definition + + Risk Symptoms Severity Mild-to-Moderate Individual Valvular Heart Disease Staging AS At risk Asymptomatic Progressive STAGE C (Asymptomatic Severe) STAGE D (Symptomatic Severe) + Severe + + Severe Asymptomatic Severe C1: normal LVEF C2: low LVEF Symptomatic Severe D1: high gradient D2: low flow, low gradient, low LVEF 69 D3: low flow, low gradient, preserved LVEF (paradoxical lowflow severe AS) AR At risk Asymptomatic Progressive MS At risk Asymptomatic Progressive MR At risk Asymptomatic Progressive TR At risk Asymptomatic Progressive Asymptomatic Severe C1: normal LVEF C2: low LVEF or dilated LVEF Asymptomatic Severe Asymptomatic Severe C1: normal LVEF C2: low LVEF & dilated LVEF Asymptomatic Severe Symptomatic Severe Symptomatic Severe Symptomatic Severe Symptomatic Severe II. INDIVIDUAL VALVULAR HEART DISEASES (VHD) A. Aortic Stenosis (AS) Most common cause: degenerative calcification of aortic cusps in adults Most common congenital defect: bicuspid aortic valve (BAV) Symptoms (dyspnea, angina, exertional syncope) are rarely present until valve orifice <1 cm 2 Death usually at 7th-8th decades, and may depend on the presence of symptoms: o If with syncope I angina: death in 3 years o If with dyspnea: death in 2 years o If with CHF: death in 1.5-2 years Physical Exam Diagnostics Therapy Pulsus parvus et tardus: weak and late pulse Low pitched midsystolic ejection murmur at 2nd R ICS Murmur may be transmitted to the apex, resembling murmur of MR (Gallavardin effect) CXR / ECG: LVH (with strain pattern on ECG) 2D Echo: calcified aortic valve with restriction in opening Avoidance of strenuous activity and competitive sports Diuretics for CHF Caution with the use of nitrates and afterload unloaders (ACEI/ARBs) as these may precipitate hypotension Statins for slower progression of leaflet calcification Intervention: Transcatheter Aortic Valve Implantation (TAVI), aortic valve replacement (surgery) B. Aortic Regurgitation (AR) Physical Exam Diagnostics Therapy Austin Flint murmur: soft low-pitched rumbling mid-to-late diastolic murmur De Musset sign: jarring of the body & bobbing of the head with each systole in severe AR Quincke’s pulse: visible capillary pulsations at the root of the nail with pressure Traube sign: booming pistol shot sound over femoral arteries Duroziez sign: to and from murmur when femoral artery is compressed Water hammer (Corrigan’s) pulse: bounding and forceful pulse, rapidly increasing and subsequently collapsing Others: widened pulse pressure, absence of A2 in severe AR ECG: LVH usually with ST depression and T wave inversion in I, aVL, V5-6 (lateral leads) 2D Echo: mosaic color flow across the aortic valve during diastole Diuretics, ACEI and vasodilators for CHF Intervention: aortic valve replacement (surgery) 70 C. Mitral Stenosis (MS) Rheumatic heart disease is the leading cause Poor prognosis for those >65 y/o, marked cardiac output depression, RV dysfunction and pulmonary hypertension Loud S1 and accentuated P2 Physical Exam Apical diastolic rumble and murmur Opening snap CXR: LAE, RAE, RVH Diagnostics ECG: LAE, RAE, RVH; atrial fibrillation in severe cases 2D ECHO: doming motion of the mitral valve (anterior leaflet) during diastole For fluid retention: sodium, restriction, diuretics For rate control: beta-blockers, non-dihydropyridine calcium channel blockers, digoxin Therapy For secondary prophylaxis of rheumatic heart disease: penicillin For prevention of stroke: warfarin (target INR 2-3) Intervention: percutaneous transseptal mitral commisurotomy (PTMC) or mitral valve replacement therapy (surgery) D. Mitral Regurgitation (MR) Physical Exam Diagnostics Therapy Soft S1; S4 in acute severe MR Apical holosystolic murmur radiating to axilla (characteristic finding) Hyperdynamic LV with brisk systolic impulse and laterally displaced apex beat CXR: LAE, LVH ECG: LAE, LVH; atrial fibrillation in severe cases 2D ECHO: mosaic color flow across the mitral valve during systole For fluid retention: sodium, restriction, diuretics For acute MR:vasodilators (decreases afterload and helps reduce severity of MR) Intervention:mitral valve repair or replacement (surgery) E. Mitral Valve Prolapse (MVP, Floppy Valve Syndrome, Barlow’s Syndrome) More common in women 15-30 years old More severe in men and >50 years old Most patients are asymptomatic Frequent finding in heritable connective tissue disease Apical mid- or late non-ejection systolic click (characteristic finding) Physical Exam High pitched late crescendo-decrescendo murmur after systolic click Murmur is accentuated by standing and strain phase of Valsalva, diminished by squatting and isometric exercises CXR / ECG: usually normal; but may have biphasic or inverted T in II, III, aVF Diagnostics (inferior leads) on ECG 2D ECHO: systolic displacement of MV leaflets (prolapse) at least 2 mm into LA superior to mitral plane IE prophylaxis for patients with prior endocarditis Therapy Symptoms: beat-blockers for palpitations; warfarin if with AF Intervention: mitral valve repair or replacement (surgery) if with severe MR F. Tricuspid Stenosis (TS) Generally rheumatic in origin; does not occur in isolation and usually associated with MS Almost always accompanied by severe TR Symptoms of right-sided CHF (ascites, edema, hepatosplenomegaly) Physical Exam Opening snap of TV ~0.06 sec after PV closure Diastolic murmur at LLSB, augmented during inspiration and reduced during expiration & strain phase of Valsalva Diagnostics ECG: RAE, RVH 2D ECHO: restriction in opening of the TV Therapy Salt restriction, bed rest and diuretics Interventions: surgery 71 G. Tricuspid Regurgitation (TR) Physical Exam Diagnostics Therapy Distended neck veins, hepatomegaly, ascites, (+) hepatojugular reflux Prominent RV pulsation along left parasternal region Carvallo sign: blowing holosystolic murmur at LPSB intensified by inspiration ECG: RAE, RVH 2D ECHO: mosaic color flow across tricuspid valve during systole Isolated TR is usually tolerated and does not require surgery Intervention: valve annuloplasty or replacement for severe cases H. Pulmonic Regurgitation (PR) Most common acquired abnormality is regurgitation from severe pulmonary arterial HPN Graham Steell murmur: high-pitched, decrescendo, diastolic blowing murmur along left sternal border Intervention: percutaneous pulmonic valve replacement for severe PR PERICARDITIS I. ETIOPATHOGENESIS Most common pathology affecting the pericardium and classified clinically and etiologically May be infectious, non-infectious (MI, uremia, neoplasia, myxedema, cholesterol, chylopericardium, trauma, aortic dissection, post-irradiation, acute idiopathic, sarcoidosis) or presumably related to hypersensitivity or autoimmunity (rheumatic fever, collagen valvular disease, drug-induced, post-cardiac injury) II. CLINICAL MANIFESTATIONS A. Acute Pericarditis (< 6 weeks) Pain resembles that of acute MI Chest pain: severe, pleuritic, may be retrosternal or left pericordial and may be referred to neck and, arms or left shoulder Pericardial pain may be relieved by sitting up and leaning forward and is intensified by lying supine PE may reveal pericardial friction rub (85%): high-pitched and is described as rasping, scratching or grating and heard most frequently at end-expiration with patient upright and leaning forward B. Chronic (Constrictive) Pericarditis (> 6 months) Results when the healing of an acute fibrinous or serofibrinous pericarditis or the resorption of a chronic pericardial effusion is followed by obliteration of the pericardial cavity with formation of granulation tissue Weakness, weight gain, fatigue, increased abdominal girth / ascites and edema Common in the Philippines: tuberculosis, malignancy and radiation-induced Kussmaul’s sign: increase in systemic venous pressure with inspiration (in normal conditions, there should be a decrease in pressure with inspiration) Pericardial knock: early diastolic sound in the left sternal border III. DIAGNOSIS AND MANAGEMENT DIAGNOSTICS Cardiac biomarkers ECG ACUTE PERICARDITIS Modest increase Subepicardial inflammation displays: Stage 1: Diffuse SST-elevation with upward concavity and PR segment depression Stage 2: ST segments normalize Stage 3: T-wave inversions Stage 4: ECG returns to CHRONIC (CONSTRICTIVE PERICARDITIS) May be normal-minimally increased Low voltage QRS complexes Diffuse flattening or inversion of T-waves Atrial fibrillation in 1/3 of patients 72 normal (weeks or months) This is in contrast with ECG findings in AMI wherein ST-elevations are convex, QRS changes occur and Twave inversion is seen within hours before the ST-segments become isoelectric Pericardial thickening Septal bounce Dilation of the IVC and hepatic veins Normal ventricular systolic function Flattening of the LV posterior wall Pericardial thickening (more accurate) Pericardial resection / pericardectomy Sodium restriction & diuretics Anti-Koch’s for TB patients Steroids (uncertain benefi) Pericardial fluid or thickening Differentiate pericarditis from MI: assessment of wall motion Echocardiography CT/MRI Pericardial fluid collection Pericardial thickening Bed rest NSAIDs, colchicine Pericardiocentesis if with tamponade Management CARDIAC TAMPONADE I. ETIOPATHOGENESIS Accumulation of fluid in the pericardial space causes increased intracardiac pressures causing limited ventricular filling and decreased cardiac output Three most common causes are neoplastic disease, idiopathic pericarditis and renal failure II. CLINICAL MANIFESTATIONS Dyspnea, orthopnea and fatigue Beck’s triad: hypotension, neck vein engorgement and muffled heart sounds Tachycardia, tachypnea and pulsus paradoxus (>10 mmHg decrease in SBP during inspiration) A. Diagnostics for Cardiac Tamponade DIAGNOSTICS 12-L ECG Chest Radiograph 2D Echocardiography COMMENTS/EXPECTED FINDINGS Low voltage QRS complexes with electrical alternans Multi-chambered cardiomegaly “water-bottle” sign Large pericardial effusion Right atrial and right ventricular diastolic collapse B. Differentials for Cardiac Tamponade CHARACTERISTIC CARDIAC TAMPONADE Clinical Features Pulsus Paradoxus +++ Jugular Veins Prominent y-descent +++ Prominent x-descent Kussmaul’s sign Third Heart Sound Pericardial Knock - CONSTRICTIVE PERICARDITIS RESTRICTIVE CMP RV MI EFFUSIVE CONSTRUCTIVE + + + +++ ++ ++ +++ ++ + +++ + + - + + +++ + - +++ ++ + - 73 Electrocardiogram Low ECG voltage Electrical Alternans Echocardiography Thick pericardium Pericardial effusion RV size Exaggerated Respiratory Variation CT-MRI Thick pericardium Equalization of Diastolic Pressure Cardiac Biopsy Helpful? ++ ++ ++ - ++ - - ++ + +++ Usually small +++ +++ Usually normal +++ Usually normal - ++ Enlarged +++ ++ +++ +++ +++ - ++ ++ No No Sometimes No No CMP: Cardiomyopathy; RVMI: right ventricular myocardial infarction IV. MANAGEMENT Emergency pericardiocentesis Tube pericardiostomy with pericardial window (for recurrent, infectious, malignant and other chronic causes) CARDIOMYOPATHY (CMP) Heterogenous group of diseases of the myocardium associated with mechanical and/or electrical dysfunction that usually (but not invariably) exhibit inappropriate ventricular hypertrophy or dilatation and are due to a variety of causes that frequently are genetic It excludes cardiac dysfunction that results from other structural heart diseases such as CAD, valvular disease or severe hypertension DILATED RESTRICTIVE HYPERTROPHY CARDIOMYOPATHY CARDIOMYOPATHY CARDIOMYOPATHY Cardiac enlargement, Endomyocardial scarring or Disproportionate resulting in impaired systolic myocardial infiltration hypertrophy, typically Pathophysiology function, HF, arrhythmia, resulting in restriction of involving the interventricular emboli ventricular filling septum more than the free wall Ejection Fraction Usually <30% 25-50% >60% LV Dimension Dilated >60mm >60mm (may be decreased) Often decreased LV Wall Thickness Decreased Normal or increased Markedly increased Atrial Size Increased Increased; may be massive Increased Valvular Regurgitation Related to annular dilation Related to endocardial Related to valve-septum involvement interaction Common First Symptoms Exertional intolerance Exertional intolerance, fluid Exertional intolerance; may retention early have chest pain Congestive Symptoms Left before right Right often predominates Left-sided congestion may develop late Viral, parasitic Amyloidosis Most common abnormality Common examples Peripartum Loeffler’s found at autopsy in young Alcohol, MAP, cocaine Endomyocardial competitive athletes who die Chemotherapy suddenly Normal LVEF: > 50% Normal LV dimension: < 55mm ATRIAL FIBRILLATION (AF) I. TYPES OF ATRIAL FIBRILLATION TYPE Lone AF First Diagnosed AF DEFINITION AF in a patient <60 years old with the absence of clinical findings of other cardiovascular disease, related pulmonary disease, or cardiac abnormalities Every patient who present with AF for the first time, irrespective of duration or 74 Paroxysmal AF Persistent AF Long-standing Persistent AF Permanent AF presence / severity of symptoms Self-terminating, usually within 48 hours Paroxysms may continue for up to 7 days 48 hour time point is important: after this, likelihood of spontaneous conversion is low Anticoagulation must be considered AF episode either lasts >7 days or require termination by cardioversion Lasted for > 1 year when it is decided to adopt a rhythm control strategy Presence of arrhythmia is accepted by the patient – rhythm control interventions are not pursued II. STROKE PREVENTION IN AF Efficacy of stroke prevention with aspirin is weak and the risk of major bleeding with aspirin is not significantly different from oral anticoagulants (OACs) Usually has two scoring systems: o CHA2DS2-VASc Score: to determine the risk of having a stroke in the presence of AF o HAS-BLED: to determine the risk of bleeding (since patients with AF will be given anticoagulants) A. CHA2DS2-VASc Score Estimates the risk of ischemic stroke in patients with non-rheumatic / non-valvular atrial fibrillation Better than CHADS2 in identifying “truly low risk” patients with AF Components and corresponding points: VARIABLE SCORE (POINTS) C Congestive HF / left ventricular dysfunction 1 H Hypertension (>140/9 mmHg) 1 A2 Age > 75years 2 D Diabetes 1 S2 Prior stroke / TIA / thromboembolism 2 V Vascular disease (prior MI, PAD, aortic plaque) 1 A Age 65-74 1 Sc Female sex 1 RISK 0: 0% 1: 1.3% 2: 2.2% 3: 3.2% 4: 4.0% 5: 6.7% 6: 9.8% 7: 9.6% 8: 12.5% 9: 15.2% B. HAS-BLED Score Bleeding risk score to aid in decision-making for thromboprophylaxis (to balance the risk of stroke versus risk of major bleeding) High risk for bleeding: HAS-BLED score > 3 (regular monitoring and correction of potentially reversible risk factors for bleeding) VARIABLE SCORE (POINTS) Hypertension (SBP > 160 mmHg) 1 Abnormal renal / liver function 1 Renal: Chronic dialysis or renal transplantation or creatinine > 200 umol/L 1 Liver: CLD, bilirubin >2x ULN with AST / ALT / Alk Phos >3x ULN Previous Stroke 1 Bleeding history or predisposition (bleeding diathesis, anemia, etc) 1 Labile INR (unstable or high INR) 1 Elderly (age >65) 1 Use of Drugs predisposing to bleeding (e.g., antiplatelets, NSAIDs) 1 Alcohol use (>8 drinks per week) 1 CLD: Chronic Liver Disease ULN: Upper Limit of Normal III. MANAGEMENT OF ATRIAL FIBRILLATION A. Drugs for Rate Control: B-blockers: metoprolol, bisoprolol, atenolol, esmolol, propranolol, carvedilol Non-dihydropyridine CCB: verapamil, diltiazem Digitalis / Digoxin Others: amiodarone, dronedarone 75 B. Pharmacological Cardioversion: If with structural heart disease: Amiodarone If without structural heart disease: Flecainide, Ibutilide, Propafenone C. Electrical Cardioversion: Used for patients with recent-onset AF (<48 hours) and with hemodynamic instability IV. ANTICOAGULATION FOR STROKE PREVENTION POPULATION Valvular AF (RHD, prosthetic valves) Non-Valvular AF + <65 years old + Lone AF Non-Valvulvar AF + CHA2DS2-VASc Score 0 Non-Valvular AF + CHA2DS2-VASc Score 1 Non-Valvular AF + CHA2DS2-VASc Score > 2 ANTITHROMBOTIC THERAPY FOR STROKE PREVENTION Warfarin only No antithrombotic therapy No antithrombotic therapy Class Iia: consider oral anticoagulant therapy (NOAC/warfarin) Class I: start oral anticoagulant therapy (NOAC or warfarin) A. Warfarin (Vitamin-K Antagonist) Considered for patients with AF with >1 stroke risk factor(s) provided there are no contraindications Superior to antiplatelets in preventing stroke Usual INR target: 2.0-3.0 B. Non-Vitamin K Oral Anticoagulants (NOACs) Non-inferior to warfarin, but with better safety profile Broadly preferably to warfarin in the vast majority of patients with non-valvular AF Assessment of renal function is mandatory for all NOACs, especially for Dabigatran Do not require dose adjustment on the basis of a specific coagulation test (in contrast to INR in warfarin) Do not have specific antidotes, and management of bleeding is supportive Not recommended in patients with severe renal impairment (creatinine clearance < 30 mL/min) DRUG MECHANISM OF ACTION DOSE Oral direct thrombin inhibitor 150 mg BID 150 mg BID superior to warfarin with same risk as warfarin to cause 110 mg BID for> age > 80, Dabigatran major bleeding concomitant interacting drugs, HAS-BLED > 3, creatinine 110 mg BID non-inferior to warfarin clearance 30-40 mL/min with fewer major bleeds (compared to warfarin) Oral direct factor Xa inhibitor 20 mg OD Rivaroxaban Non-inferior to warfarin in 15 mg OD if: HAS-BLED > 3, preventing stroke creatinine clearance 30-49 mL/min 5 mg BID Apixaban Oral direct factor Xa inhibitor 2.5 mg BID if : age > 80 years, weight < 60 kg, or creatinine > 133 umol/L PERIPHERAL ARTERY DISEASE (PAD) I. ETIOPATHOGENESIS Clinical disorder characterized by stenosis or occlusion in the aorta or arteries of the limbs Atherosclerosis is the leading cause of PAD in patients >40 years old II. CLINICAL MANIFESTATIONS A. History and Symptoms More than half of patients with PAD are actually asymptomatic, though some may present with slow gait Most common symptoms: intermittent claudication (pain, ache, cramp, numbness, or sense of fatigue in the muscles which occurs during exercise and is relieved by rest) Other symptoms are rest pain or feeling of coldness or numbness in the feet and toes 76 B. Physical Examination Decreased or absent pulses distal to obstruction Bruits over narrowed artery Muscle atrophy, hair loss, thickened nails, smooth and shiny skin Reduced skin temperature Pallor, cyanosis,, ulcers or gangrene III. DIAGNOSIS DIAGNOSTICS ABI Assessment by Doppler Other Non-Invasive Tests COMMENTS / EXPECTED FINDINGS ABI: ratio of ankle to brachial artery pressure o >1.0: normal individuals o <0.9: in patients with PAD o <0.5: signifies severe ischemia (at risk for critical limb ischemia) Segmental pressure measurements: presence of pressure gradients between sequential cuffs signify stenosis Segmental pulse volume recordings: amplitude of pulse volume contour becomes blunted in significant PAD Duplex ultrasonography: images and detects stenosis Transcutaneous oximetry Treadmill testing: assesses functional limitations objectively IV. MANAGEMENT A. Non-Pharmacologic Management Goals: reduce the risk of associated CV events, improve limb symptoms, prevent progression to critical ischemia, and preserve limb viability Risk factor modification: cigarette smoking cessation, BP control Supportive: feet care, elastic supports should be avoided, regular exercise (walk until nearly maximum claudication discomfort is experience, and then rest until symptoms resolve before resuming ambulation) Revascularization is usually indicated for patients with disabling, progressive or severe symptoms despite medical therapy and for those critical limb ischemia B. Physical Examination Antiplatelet Therapy Anticoagulant Therapy ACE-Inhibitors Statins Cilostazol Pentoxifylline Aspirin and clopidogrel dual therapy is not more effective than aspirin alone in reducing CV morbidity and mortality in patients with PAD Not indicated to improve outcomes in patients with chronic PAD Reduce CV risks in patients with PAD Target LDL <100 mg/dL Increases claudication distance by 40-60% and improves measured quality of life Contraindicated in patients with CHF Increases blood flow to the microcirculation and enhances tissue oxygenation COR PULMONALE I. ETIOPATHOGENESIS Often referred to as “pulmonary heart disease” Defined as altered RV structure and/or function in the context of chronic lung disease and is triggered by the onset of pulmonary hypertension Acute Cor Pulmonale Acute RV dilatation and failure occurs (e.g., massive pulmonary embolism) but RV does not hypertrophy Chronic Cor Pulmonale More slowly evolving and progressive pulmonary hypertension leads to both RV hypertrophy and dilation II. CLINICAL MANIFESTATIONS Dyspnea: most common symptom and occurs due to increased work of breathing 77 Tussive or effort-related syncope happens due to inability of RV to deliver adequate blood volume to the LV Abdominal pain and ascites: happens due to backflow from right-sided HF Orthopnea and PND: uncommon and occurs only with concurrent LV failure RV heave: points to RV volume and pressure overload Carvallo’s sign: increase in the intensity of the holosystolic murmur of tricuspid regurgitation with inspiration Cyanosis: a late finding and is secondary to low cardiac output III. DIAGNOSIS DIAGNOSTICS 12-L ECG Chest Radiography 2D Echocardiography Spirometry CT scan COMMENTS / EXPECTED FINDINGS P pulmonale (p waves > 2.5 mV in leads II and/or V1) Right axis deviation and RV hypertrophy Enlargement of main pulmonary artery, hilar vessels & descending right pulmonary artery Right-sided chamber enlargement with dysfunction; pulmonary hypertension Identifies obstructive and restrictive parenchymal diseases Identifies thromboembolic diseases, interstitial diseases IV. MANAGEMENT Target the underlying pulmonary disease to decrease the underlying pulmonary valvular resistance and lessen RV afterload Non-invasive mechanical ventilation, bronchodilators and correction of respiratory acidosis Correction of infection, anemia and polycythemia and other extra-cardiac problems Pulmonary vasodilators: modest reduction of pulmonary pressure and RV afterload 78