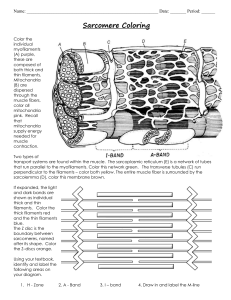
The Cytoskeleton Cells contain elaborate arrays of protein fibers that serve such functions as establishing cell shape, providing mechanical strength, and locomotion. These fibers participate in chromosome separation in mitosis and meiosis and intracellular transport of organelles. The cytoskeleton is made up of three kinds of protein filaments: Actin filaments (also called microfilaments), Intermediate filaments, and Microtubules. Actin Filaments Monomers of the protein actin polymerize to form long, thin fibers. These are about 8 nm in diameter and, being the thinnest of the cytoskeletal filaments, are also called microfilaments (in skeletal muscle fibers they are called "thin" filaments). Some functions of actin filaments are: • form a band just beneath the plasma membrane that o provides mechanical strength to the cell o links transmembrane proteins (e.g., cell surface receptors) to cytoplasmic proteins o pinches dividing animal cells apart during cytokinesis • generate cytoplasmic streaming in some cells • generate locomotion in cells such as white blood cells and the amoeba • interact with myosin ("thick") filaments in skeletal muscle fibers to provide the force of muscular contraction Intermediate Filaments These cytoplasmic fibers average 10 nm in diameter (and thus are "intermediate" in size between actin filaments (8 nm) and microtubules (25 nm) (as well as of the thick filaments of skeletal muscle fibers). There are several types of intermediate filament, each constructed from one or more proteins characteristic of it. • keratins are found in epithelial cells and also form hair and nails; • nuclear lamins form a meshwork that stabilizes the inner membrane of the nuclear envelope; • neurofilaments strengthen the long axons of neurons; • vimentins provide mechanical strength to muscle (and other) cells. Despite their chemical diversity, intermediate filaments play similar roles in the cell: providing a supporting framework within the cell. For example, the nucleus in epithelial cells is held within the cell by a basketlike network of intermediate filaments made of keratins. Different kinds of epithelia use different keratins to build their intermediate filaments. Over 20 different kinds of keratins have been found, although each kind of epithelial cell may use no more than 2 of them. Up to 85% of the dry weight of squamous epithelial cells can consist of keratins. Microtubules Microtubules are straight, hollow cylinders whose wall is made up of a ring of 13 "protofilaments" and have a diameter of about 25 nm. They are variable in length but can grow 1000 times as long as they are wide. They are built by the assembly of dimers of alpha tubulin and beta tubulin. Microtubules are found in both animal and plant cells. In plant cells, microtubules are created at many sites scattered through the cell. In animal cells, the microtubules originate at the centrosome. The attached end is called the minus end; the other end is the plus end. Microtubules grow at the plus end by the polymerization of tubulin dimers (powered by the hydrolysis of GTP), and shrink by the release of tubulin dimers (depolymerization) at the same end. They participate in a wide variety of cell activities. Most involve motion. The motion is provided by protein "motors" that use the energy of ATP to move along the microtubule. ActinThe monomeric unit of actin is called G-actin (globular actin) and the polymer is known as Factin (filamentous actin). Filaments of F actin comprise the smallest filaments of cells known as microfilaments. Actin filaments extend when A TP-actin monomers are preferentially incorporated at the barbed end. As the filament matures, A TP bound in the central cleft of actin is hydrolysed, phosphate is released and the resulting ADP-actin filament is disassembled by loss of monomers from the pointed end. The released ADP-actin monomers then undergo nucleotide exchange to generate A TP-actin monomers that can be used for new rounds of polymerisation. This A TP- hydrolysis-driven, directional filament- growth is called actin treadmilling. Actin is the most abundant intracellular protein in most eu karyotic cells. In muscle cells, for example, actin comprises 10 percent by weight of the total cell protein; even in non- muscle cells, actin makes up 1–5 percent of the cellular protein. The cytosolic concentration of actin in non-muscle cells ranges from 0.1 to 0.5 mM; in special structures such as microvilli, however, the local actin concentration can be 5 mM. Actin Polymerization -The in vitro polymerization of G-actin proceeds in three sequential phases. Nucleation -The first nucleation phase is marked by a lag period in which G-actin aggregates into short, unstable oligomers. When the oligomer reaches a certain length (three or four subunits), it can act as a stable seed, or nucleus. Elongation -In the second elongation phase nucleus rapidly increases in length by the addition of actin monomers to both of its ends. As F-actin filaments grow, the concentration of G-actin monomers decreases until equilibrium is reached between filaments and monomers. Steady-state phase-In this third steady-state phase, G-actin monomers exchange with subunits at the fil- ament ends, but there is no net change in the total mass of fil- aments. Regulation of actin polymerization by actin binding Proteins Nucleation regulation-The first stage in de novo filament formation is nucleation. Polymerisation is energetically unfavourable until there is a nucleus of three associating monomers and in vitro this stage of filament formation is denoted the lag phase. In vivo, ABPs are crucial to ensure the rapid nucleation of filaments and these essentially function to remove the lag period. New filaments can form de novo, from the side of existing filaments, or by severing an existing filament. Different proteins function to promote each of these modes of nucleation. The Arp2/3 complex can nucleate filaments from the side of existing filaments. This is important to allow the dendritic branching that is found at the leading edge of motile cells. Arp2 and Arp3 molecules themselves have a similar tertiary structure to actin itself, and when the Arp2/3 complex binds G-actin the effect is to generate a stable trimer and nucleus for the growth of a filament. Arp2/3 then acts as a pointed-end-capping protein that encourages rapid growth of the filament from its barbed end. Whereas the Arp2/3 complex can nucleate new filaments in vitro, it is likely that its activity in vivo is enhanced by functional interactions with other proteins, most importantly the W ASP and SCAR/W A VE proteins. Actin polymerisation is also nucleated by the formin proteins. These proteins have only recently come under close scrutiny and there is still much to be understood about their mechanism of action in cells. Inhibition of Actin Assembly by Thymosin. Because of its abundance in the cytosol and ability to bind ATP–G-actin (but not F-actin), thymosin is considered to be the main actin-sequestering protein in cells. A small protein (5000 MW), thymosin binds ATP–G-actin in a 1:1 complex. The binding of thymosin blocks the ATP-binding site in G-actin, thereby preventing its polymerization. Promotion of Actin Assembly by Profilin Another cytosolic protein, profilin (15,000 MW), also binds ATP-actin monomers in a stable 1:1 complex. First, profilin promotes the assembly of actin filaments by acting as a nucleotideexchange factor. Profilin is the only actin-binding protein that allows the exchange of ATP for ADP. When G-actin is complexed with other proteins, ATP or ADP is trapped in the ATPbinding cleft of actin. However, because profilin binds to G-actin at a site opposite the ATPbinding cleft, it can recharge ADP-actin monomers re- leased from a filament, thereby replenishing the pool of ATP- actin Filament-Binding Severing Proteins Create New Actin Ends A second group of proteins, which bind to actin filaments, control the length of actin filaments by breaking them into shorter fragments and generating new filament ends for polymerization Because the actin concentration in a cell favors the formation of filaments, the breakdown of existing actin filaments and filament networks requires the assistance of severing proteins such as gelsolin and cofilin Note- Filaments are controlled by several mechanisms. Filament length is controlled by capping proteins. Barbed end cappers such as capping protein, gelsolin and tensin block addition of new monomers, so acting to decrease the overall length of the filament. In addition, gelsolin can sever actin filaments, thereby rapidly increasing actin dynamics.. The best characterised proteins that drive depolymerisation are the actindepolymerizing factor (ADF) and the cofilin family members. This ubiquitous protein is highly conserved and plays a central role in actin turnover. It binds to ADP-F-actin and promotes dissociation of ADP-actin from the pointed end of the filament. ADF/cofilin also associates with AIP-1 (actin-interacting protein1). This interaction appears to increase the depolymerising activity of cofilin. The complex also promotes barbed end capping, although the details of this mechanism are not yet well understood. Actin-Capping Proteins Stabilize F-Actin Another group of proteins can cap the ends of actin filaments but, unlike severing proteins, cannot break filaments to create new ends. One such protein, CapZ, binds the + ends of actin filaments independently of Ca2+and prevents the addition or loss of actin subunits from the + end. Capping by this protein is inhibited by PIP2, suggesting that its activity is regulated by the same signaling pathways that control cofilin and profilin. Tropomodulin, which is unrelated to CapZ in sequence, caps the (-) ends of actin filaments. Its capping activity is enhanced in the presence of tropomyosin, which suggests that the two proteins function as a complex to stabilize a filament Intracellular Movements and Changes in Cell Shape Are Driven by Actin Polymerization Most infections are spread by bacteria or viruses that are liberated when an infected cell lyses. However, some bacteria and viruses escape from a cell on the end of a polymerizing actin filament. Examples include Listeria monocytogenes, a bacterium that can be transmitted from a pregnant woman to the fetus, and vaccinia, a virus related to the smallpox virus. When such organisms infect mammalian cells, they move through the cytosol at rates approaching 11 nm/min. Fluorescence microscopy revealed that a meshwork of short actin filaments follows a moving bacterium or virus like the plume of a rocket exhaust . These observations suggested that actin generates the force necessary for movement. Motor protein Myosin- Myosins Are a Large Superfamily of Mechanochemical Motor Proteins . Eight members of the myosin gene family have been identified by genomic analysis . Three family mem- bers—myosin I, myosin II, and myosin V—are present in nearly all eukaryotic cells and are the best understood. Although the specific activities of these myosins differ, they all function as motor proteins. As already noted, myosin II powers muscle contraction, as well as cytokinesis. Myosins I and V take part in cytoskeleton–membrane interactions, such as the transport of membrane vesicles. Researchers are currently uncovering the activities of the remaining myosins. Genetic analysis has revealed that myosins VI, VII, and XV have functions associated with hearing and hair cell stereocilia structure. Plants do not have the same myosins as animal cells. Three myosins (VII, XI, and XIII) are exclusively expressed in plants. Myosin XI, which may be the fastest myosin of all, is implicated in the cytoplasmic streaming seen in green algae and higher plants All myosins consist of one or two heavy chains and several light chains, which generally have a regulatory function. A characteristic head, neck, and tail domain organization is found in all myosin heavy chains. Myosin II and myosin V are dimers in which alpha-helical sequences in the tail of each heavy chain associate to form a rodlike coiled-coil structure. In contrast some myosins, including myosin I, are monomers because their heavy chains lack this alpha-helical sequence. All myosin head domains have ATPase activity and in conjunction with the neck domain couple ATP hydrolysis to movement of a myosin molecule along an actin filament via a common mechanism involving cyclical binding and hydrolysis of ATP and attachment/detachment of myosin and actin . Actin and Myosin II Non-muscle Cells Nonmuscle cells contain prominent contractile bundles com- posed of actin and myosin II filaments. In epithelial cells, contractile bundles are most commonly found as a circumferential belt, which encircles the inner sur face of the cell at the level of the adherens junction. Stress fibers, which are seen along the ventral surfaces of cells cultured on artificial (glass or plastic) sur- faces or in extracellular matrices, are a second type of contractile bundle. A third type contractile of bundle, referred to as a contractile ring, is a transient structure that assembles at the equator of a dividing encircling the cell, cell midway between the poles of the spindle. As division of the cytoplasm (cytokinesis) proceeds, the diameter of the contractile ring de- creases; so the cell is pinched into two parts by a deepening cleavage furrow. Skeletal Muscle Cells Muscle cells have evolved to carry out one highly specialized function—contraction. A typical skeletal muscle cell, called a myofiber, is cylindrical, large (1– 40 mm in length and 10–50 micrometer in width), and multinucleated (containing as many as 100 nuclei). The cytoplasm is packed with a regular repeating array of filament bundles organized into a specialized structure called a sarcomere. A chain of sarcomeres, each about 2 micrometer long in resting muscle, constitutes a myofibril. The sarcomere is both the structural and the functional unit of skeletal muscle. During contraction, the sarcomeres are shortened to about 70 percent of their uncontracted, resting length Electron microscopy and biochemical analysis have shown that each sarcomere contains two types of filaments: thick filaments, composed of myosin II, and thin filaments, containing actin. Muscles contraction – In the presence of ATP and Ca2+, the myosin heads extending from the thick filaments walk toward the + ends of the thin filaments. Because the thin filaments are anchored at the Z disks (purple), movement of myosin pulls the actin filaments toward the center of the sarcomere, shortening its length in the contracted state . During these cyclical interactions, also called the cross-bridge cycle, the hydrolysis of ATP is coupled to the movement of a myosin head toward the Z disk, which corresponds to the (+) end of the thin filament. Because the thick filament is bipolar, the action of the myosin heads at opposite ends of the thick filament draws the thin filaments toward the center of the thick filament and there- fore toward the center of the sarcomere . This movement shortens the sarcomere until the ends of the thick filaments about the Z disk or the (-) ends of the thin filaments overlap at the center of the A band. Contraction of an intact muscle results from the activity of hundreds of myosin heads on a single thick filament, amplified by the hundreds of thick and thin filaments in a sarcomere and thousands of sarcomeres in a muscle fiber. Regulation of Skeletal Muscle contraction Like many cellular processes, skeletal muscle contraction is initiated by an increase in the cytosolic Ca2+ concentration. As described in Chapter 7, the Ca2+ concentration of the cytosol is normally kept low, below 0.1 micro molar. In non-muscle cells, Ca2+ ATPases in the plasma membrane maintain this low concentration. In contrast, in skeletal muscle cells, a low cytosolic Ca2+ level is maintained primarily by a unique Ca2+ ATPase that continually pumps Ca2+ ions from the cytosol into the sarcoplasmic reticulum (SR), a specialized endoplasmic reticulum in the muscle-cell cytosol . This activity establishes a reservoir of Ca2+ in the SR. The arrival of a nerve impulse at a neuromuscular junction leads to the opening of voltagegated Ca2+ channels in the SR membrane . The ensuing release of Ca2+from the SR raises the cytosolic Ca2+ concentration surrounding myofibrils sufficiently to trigger contraction. In skeletal muscle, the cytosolic Ca2+ concentration influences the interaction of four accessory proteins with actin thin filaments. The position of these proteins on the thin filaments in turn controls myosin–actin interactions. Tropomyosin (TM) is a rope-like molecule, about 40 nm in length; TM molecules are strung together head to tail, forming a continuous chain along each actin thin filament. Associated with tropomyosin is troponin (TN), a complex of the three subunits, TN-T, TN-I, and TN-C. Troponin-C is the calcium-binding subunit of troponin. Similar in sequence to calmodulin and the myosin light chains, TN-C controls the position of TM on the surface of an actin filament through the TN-I and TN-T subunits. Microtubules Microtubules are major components of the cytoskeleton of eukaryotic cells, providing structural support, transport within the cell, and functions necessary for segregation of DNAs during cell division Microtubules are formed from molecules of tubulin, each of which is a heterodimer consisting of two closely related and tightly linked globular polypeptides called a-tubulin and b-tubulin. Although tubulin is present in virtually all eucaryotic cells, the most abundant source for biochemical studies is the vertebrate brain. Tubulins Tubulins belong to an ancient family of GTPases that polymerize to form microtubules .Tubulin proteins are the monomeric building blocks of eukaryotic microtubules . Bacterial (TubZ) and archaeon (FtsZ) equivalents are known. The α-tubulin and β-tubulin proteins polymerize to make microtubule structures in the cytoplasm of cells.. Dimerization of the α-tubulin and β-tubulin proteins is necessary for polymerization and requires that the subunits bind to GTP. Microtubules only grow in one direction. β- tubulin is found on the plus end of the tubule (growth end = plus end) and α-tubulin is exposed on the other end (non-growth end = minus end). Dimers of α-tubulin/β-tubulin are incorporated into growing microtubules in this orientation. If a dimer is bound to GDP instead of GTP, it tends to be unstable and fall apart, whereas those bound to GTP stably assemble into microtubules. Microtubules, like actin microfilaments, exhibit both structural and functional polarity. Dimeric a – b tubulin subunits interact end-to-end to form protofilaments, which associate laterally into microtubules. Microtubules exhibit structural polarity. Virtually every microtubule in a cell is a simple tube, a singlet microtubule, built from 13 protofilaments. In rare cases, singlet microtubules contain more or fewer protofila- ments; for example, certain microtubules in the neurons of nematode worms contain 11 or 15 protofilaments. In addi- tion to the simple singlet structure, doublet or triplet micro- tubules are found in specialized structures such as cilia and flagella (doublet microtubules) and centrioles and basal bod- ies (triplet microtubules). Each doublet or triplet contains one complete 13-protofilament microtubule (A tubule) and one or two additional tubules (B and C) consisting of 10 protofilaments Polymerization Polymerization of α- tubulin and β-tubulin to form microtubules occurs after a nucleating event. Individual units get arranged in microtubule organizing centers (MTOCs), an example of which is the centrosome. Centrosomes are focal points of connection of microtubules. Basal bodies of cilia and flagella also help to organize microtubules. Microtubule assembly comprises three steps: (1) proto-filaments assemble from a – b –tubulin subunits, (2) protofilaments associate to form the wall of the microtubule, and (3) the addition of more subunits to the ends of the proto- filaments elongates the microtubule A large number of proteins influence the assembly and stability of microtubules and their association with other cell structures. These proteins are collectively called microtubuleassociated proteins (MAPs) because most copurify with microtubules isolated from cells. Tubulin polymerization has several properties in common with the polymerization of actin to form microfilaments. First, Assembly and disassembly of microtubules depends on the critical concentration, Cc, of a – b –tubulin subunits. Above the Cc, microtubules assemble; below the Cc, microtubules disassemble.. Second, the nucleotide, either GTP or GDP, bound to the b tubulin causes the critical concentration (Cc) for assembly at the (+) and (-) ends of a microtubule to differ. Microtubules exhibit two dynamic phenomena that are pronounced at tubulin concentrations near the Cc: (1) tread- milling, the addition of subunits at one end and their loss at the other end, and (2) dynamic instability, the oscillation between lengthening and shortening . The balance between growth and shrinkage of unstable microtubules depends on whether the exchangeable GTP bound to b -tubulin is present on the + end or whether it has been hydrolyzed to GDP. Microtubule-associated proteins (MAPs) organize microtubules and affect their stability. Some MAPs prevent or promote cytosolic microtubule depolymerization; other MAPs organize microtubules into bundles or cross-link them to membranes and intermediate filaments or both. Because microtubule assembly is nucleated from MTOCs, the (-) end of most microtubules is adjacent to the MTOC and the (+) end is distal. Kinesins and dyneins As noted, kinesins and dyneins navigate in cells on microtubule tracks Most kinesins move in the direction of the synthesis of the microtubule (+ end movement), which is generally away from the cell center and the opposite direction of movement of dyneins, which are said to do retrograde transport toward the cell center. Both proteins provide movement functions necessary for the processes of mitosis and meiosis. These include spindle formation, chromosome separation, and shuttling of organelles, such as the mitochondria, Golgi apparatuses, and vesicles. Kinesins are comprised of two heavy chains and two light chains. The head motor domains of heavy chains (in the feet) use energy of ATP hydrolysis to do mechanical work for the movement along the microtubules. There are at least fourteen distinct kinesin families and probably many related ones in addition. Dyneins are placed into two groups - cytoplasmic and axonemal. Dyneins are more complex in structure than kinesins with many small polypeptide units. Notably, plants do not have dynein motor proteins, but do contain kinesins. Intermediate Filaments Intermediate filaments are found in nearly all animals but not in in plants and fungi. The association of intermediate filaments with the nuclear and plasma membranes suggests that their principal function is structural In epithelium, for in- stance, intermediate filaments provide mechanical support for the plasma membrane where it comes into contact with other cells or with the extracellular matrix. In epidermal cells (outer layer of skin) and the axons of neurons , intermediate filaments are at least 10 times as abundant as microfilaments or microtubules, the other components of the cytoskeleton. Unlike microtubules and microfilaments, intermediate filaments are assembled from a large number of different IF proteins. These proteins are divided into four major types based on their sequences and tissue distribution. The lamins are expressed in all cells, whereas the other types are expressed in specific tissues. Lamins The most ubiquitous group of IFs are the lamins. In contrast with the cytosolic location of the other four classes of IF proteins, lamins are found exclusively in the nucleus. keratins Epithelial cells express acidic and basic keratins. They associate in a 1:1 ratio to form heterodimers, which assemble into heteropolymeric keratin filaments; neither type alone can assemble into a keratin filament. The keratins are the most diverse classes of IF proteins, with a large number of keratin isoforms being expressed. These isoforms can be divided into two groups: about 10 keratins are specific for “hard” epithelial tissues, which give rise to nails, hair, and wool; and about 20, called cytokeratins, are more generally found in the epithelia that line internal body cavities. Each type of epithelium always expresses a characteristic combi- nation of acidic and basic keratins. Type III IF proteins Four proteins are classified as type III IF proteins. Unlike the keratins, the type III proteins can form both homo- and hetero polymeric IF filaments. The most widely distributed of all IF proteins is vimentin, which is typically expressed in leukocytes, blood vessel endothelial cells, some epithelial cells, and mesenchymal cells such as fibroblasts . Vimentin filaments help support cellular membranes. Vimentin networks may also help keep the nucleus and other organelles in a de- fined place within the cell. Vimentin is frequently associated with microtubules and, as noted earlier, the network of vimentin filaments parallels the microtubule network . The other type III IF proteins have a much more limited distribution. Desmin filaments in muscle cells are re- sponsible for stabilizing sarcomeres in contracting muscle. Glial fibrillary acidic protein forms filaments in the glial cells that surround neurons and in astrocytes. Peripherin is found in neurons of the peripheral nervous system, but little is known about it. Neurofilaments (NFs), The core of neuronal axons is filled with neurofilaments (NFs), each a heteropolymer composed of three polypeptides— NF-L, NF-M, and NF-H—which differ greatly in molecular weight. Neurofilaments are responsible for the radial growth of an axon and thus deter- mine axonal diameter, which is directly related to the speed at which it conducts impulses. Assembly of intermediate filaments IF proteins form parallel dimers with a highly conserved coiled-coil core domain and globular tails and heads, which are variable in length and sequence. (b) A tetramer is formed by antiparallel, staggered side-by-side aggregation of two identical dimers. (c) Tetramers aggregate end-to-end and laterally into a protofibril. In a mature filament, consisting of four protofibrils, the globular domains form beaded clusters on the surface. The assembly of intermediate filaments probably proceeds through several intermediate structures, which associate by lateral and end-to-end interactions EXTRA MATERIAL Actin Cellular Action Examples of actin action at the cellular level include cell motility, cytokinesis, intracellular transport of vesicles and organelles, and cell shape. Each actin monomer is bound to a molecule of ATP or ADP and the presence of one of these is essential for proper G-actin functioning. Figure 2.102 - Attachment of actin at the cell membrane complex known as the adherens junction Wikipedia The role of ATP In the monomer, actin is more commonly bound to ATP, whereas in the filaments, it is typically bound to ADP. Actin is an inefficient ATPase, breaking the molecule down slowly, but the catalysis speeds up as much as 40,000 fold when the monomer begins to polymerize. Actin also has a binding site for divalent cations - either calcium or magnesium. FActin binds to structural proteins at the adherens junction (Figure 2.102). These include αactinin, vinculin (provides a membrane connection and connections to the catenins and cadherin). Motor proteins From the transport of materials within a cell to the process of cytokinesis where one cell splits into two in mitosis, a cell has needs for motion at the molecular level. Secretory vesicles and organelles must be transported. Chromosomes must be separated in mitosis and meiosis. The proteins dynein and kinesin (Figure 2.106) are necessary for intracellular movement. These motor proteins facilitate the movement of materials inside of cells along microtubule “rails”. These motor proteins are able to move along a portion of the cytoskeleton by converting chemical energy into motion with the hydrolysis of ATP. An exception is flagellar rotation, which uses energy provided from a gradient created by a proton pump. Figure 2.107 - Kinesin. “Feet” are at the top. Figure 2.108 - Nomenclature of dynein. The “feet” of Figure 2.105 are the stalk and globular head of the structure here. Wikipedia Movie 2.4 The motor protein kinesin walking down a microtubule. Image used with permission (Public Domain; zp706). Myosin An important group of motor proteins in the cell is the myosins. Like kinesins and dyneins, myosins use energy from hydrolysis of ATP for movement. In this case, the movement is mostly not along microtubules, but rather along microfilaments comprised of a polymer of actin (F-actin). Movement of myosin on actin is best known as the driving force for muscular contraction. Myosins are a huge family of proteins, all of which bind to actin and all of which involve motion. Eighteen different classes of myosin proteins are known. Figure 2.109 - Dynein in an axoneme Wikipedia Myosin II is the form responsible for generating muscle contraction. It is an elongated protein formed from two heavy chains with motor heads and two light chains. Each myosin motor head binds actin and has an ATP binding site. The myosin heads bind and hydrolyze ATP. This hydrolysis produces the energy necessary for myosin to walk toward the plus end of an actin filament. Figure 2.110 - Actin filament anatomy Wikipedia Non-muscle myosin IIs provide contraction needed to power the action of cytokinesis. Other myosin proteins are involved in movement of non-muscle cells. Myosin I is involved in intracellular organization. Myosin V performs vesicle and organelle transport. Myosin XI provides movement along cellular microfilament networks to facilitate organelle and cytoplasmic streaming in a particular direction. Structure Myosins have six subunits, two heavy chains and four light chains. Myosin proteins have domains frequently described as a head and a tail (Figure 2.111). Some also describe an intermediate hinge region as a neck. The head portion of myosin is the part that binds to actin. It uses energy from ATP hydrolysis to move along the actin filaments. In muscles, myosin proteins form aggregated structures referred to as thick filaments. Movements are directional. Figure 2.111 - Myosin protein anatomy Wikipedia Structural considerations of muscular contraction Before we discuss the steps in the process of muscular contraction, it is important to describe anatomical aspects of muscles and nomenclature. There are three types of muscle tissue - skeletal (striated), smooth, and (in vertebrates) cardiac. We shall concern ourselves mostly here with skeletal muscle tissue. Muscles may be activated by the central nervous system or, in the case of smooth and cardiac muscles, may contract involuntarily. Skeletal muscles may be slow twitch or fast twitch. Figure 2.112 - Structural components of muscle. Wikipedia Sarcomeres Sarcomeres are described as the basic units comprising striated muscles and are comprised of thick (myosin) and thin (actin) filaments and a protein called titin. The filaments slide past each other in muscular contraction and then backwards in muscular relaxation. They are not found in smooth muscles. Under the microscope, a sarcomere is the region between two Z-lines of striated muscle tissue (Figure 2.112). The Z-line is the distinct, narrow, dark region in the middle of an I-band. Within the sarcomere is an entire Aband with its central H-zone. Within the Hzone are located tails of myosin fibers, with the head pointed outwards from there projecting all the way to the I-band. The outside of the Aband is the darkest and it gets lighter moving towards the center. Within the Iband are located thin filaments that are not occupied with thick myosin filaments. The Aband contains intact thick filaments overlaying thin filaments except in the central H zone, which contains only thick filaments. In the center of the H-zone is a line, known as the M-line. It contains connecting elements of the cellular cytoskeleton. In muscular contraction, myosin heads walk along pulling their tails over the actin thin filaments, using energy from hydrolysis of ATP and pulling them towards the center of the sarcomere. Figure 2.113 - Skeletal muscle longitudinal section. Wikipedia Sarcolemma The sarcolemma (also known as the myolemma) is to muscle cells what the plasma membrane is to other eukaryotic cells - a barrier between inside and outside. It contains a lipid bilayer and a glycocalyx on the outside of it. The glyocalyx contains polysaccharides and connects with the basement membrane. The basement membrane serves a s scaffolding to connect muscle fibers to. This connection is made by transmembrane proteins bridging the actin cytoskeleton on the inside of the cell with the basement membrane on the outside. On the ends of the muscle fibers, each sarcolemma fuses with a tendon fiber and these, in turn, adhere to bones. Sarcoplasmic reticulum The sarcoplasmic reticulum (Figure 2.114) is a name for the structure found within muscle cells that is similar to the smooth endoplasmic reticulum found in other cells. It contains a specialized set of proteins to meet needs unique to muscle cells. The organelle largely serves as a calcium “battery,” releasing stored calcium to initiate muscular contraction when stimulated and taking up calcium when signaled at the end of the contraction cycle. It accomplishes these tasks using calcium ion channels for release of the ion and specific calcium ion pumps to take it up. Movement direction All myosins but myosin VI move towards the + end (the growing end) of the microfilament. The neck portion serves to link the head and the tail. It also a binding site for myosin light chain proteins that form part of a macromolecular complex with regulatory functions. The tail is the point of attachment of molecules or other “cargo” being moved. It can also connect with other myosin subunits and may have a role to play in controlling movement. Figure 2.114 - Anatomy of a muscle fiber Wikipedia Muscular contraction The sliding filament model has been proposed to describe the process of muscular tension/contraction. In this process a repeating set of actions slide a thin actin filament over a thick myosin filament as a means of creating tension/ shortening of the muscle fiber. Steps in the process occur as follows: A. A signal from the central nervous system (action potential) arrives at a motor neuron, which it transmits towards the neuromuscular junction (see more on the neurotransmission part of the process HERE) Figure 2.115 - 1. Activation of a muscle cell by release of calcium (step H) Wikipedia B. At the end of the axon, the nerve signal stimulates the opening of calcium channels at the axon terminus causing calcium to flow into the terminal. C. Movement of calcium into the axon of the nerve causes acetylcholine (a neurotransmitter) in synaptic vesicles to fuse with the plasma membrane. This causes the acetylcholine to be expelled into the synaptic cleft between the axon and the adjacent skeletal muscle fiber. Figure 2.116 - 2. Calcium binding by troponin allows myosin to access actin sites (I). Wikipedia D. Acetylcholine diffuses across the synapse and then binds to nicotinic acetylcholine receptors on the neuromuscular junction, activating them. E. Activation of the receptor stimulates opening gates of sodium and potassium channels, allowing sodium to move into the cell and potassium to exit. The polarity of the membrane of the muscle cell (called a sarcolemma - Figure 2.111) changes rapidly (called the end plate potential). Figure 2.117 - 3. ATP cleavage by myosin allows actin attachment (J) Wikipedia F. Change in the end plate potential results in opening of voltage sensitive ion channels specific for sodium or potassium only to Figure 2.117 - 3. ATP cleavage by myosin allows actin attachment (J) Wikipediaopen, creating an action potential (voltage change) that spreads throughout the cell in all directions. G. The spreading action potential depolarizes the inner muscle fiber and opens calcium channels on the sarcoplasmic reticulum (Figure 2.115). H. Calcium released from the sarcoplasmic reticulum binds to troponin on the actin filaments (Figure 2.115). I. Troponin alters the structure of the tropomyosin to which is it bound. This causes tropomyosin to move slightly, allowing access to myosin binding sites on the microfilament (also called thin filament) that it was covering (Figure 2.116). J. Myosin (bound to ATP) cleaves the ATP to ADP and Pi, which it holds onto in its head region and then attaches itself to the exposed binding sites on the thin filaments causing inorganic phosphate to be released from the myosin followed by ADP (Figure 2.117). Figure 2.118 - 4. Release of Pi causes myosin hinge to bend. Thin filament pulled left (K). Wikipedia K. Release of ADP and Pi is tightly coupled to a bending of the myosin hinge, resulting in what is called the power stroke. This causes the thin filament to move relative to the thick fibers of myosin (Figures 2.118 & 2.119). Figure 2.119 - 5. Release of ADP favors further bending of hinge and movement of thin filament leftward (K). Wikipedia L. Such movement of the thin filaments causes the Z lines to be pulled closer to each other. This results in shortening of the sarcomere as a whole (Figure 2.122) and narrowing of the I band and the H zones (Figure 2.123). M. If ATP is available, it binds to myosin, allowing it to let go of the actin (Figures 2.120 & 2.121). If ATP is not available, the muscle will remain locked in this state. This is the cause of rigor mortis in death - contraction without release of muscles . Figure 2.120 - When ATP is present, it binds to myosin (M). Wikipedia N. After myosin has bound the ATP, it hydrolyzes it, producing ADP and Pi, which are held by the head. Hydrolysis of ATP resets the hinge region to its original state, unbending it. This unbent state is also referred to as the cocked position. O.If tropomyosin is still permitting access to binding sites on actin, the process repeats so long as ATP is available and calcium remains at a high enough concentration to permit it to bond to troponin. Figure 2.121 - Binding of ATP favors release of myosin from actin site (N) Wikipedia Relaxation of the muscle tension occurs as the action potential in the muscle cell dissipates. This happens because all of the following things happen 1) the nerve signal stops; 2) the neurotransmitter is degraded by the enzyme acetylcholinesterase; and 3) the calcium concentration declines because it is taken up by the sarcoplasmic reticulum. Figure 2.122 - Sarcomere Anatomy Wikipedia It should be noted that the sarcoplasmic reticulum is always taking up calcium. Only when its calcium gates are opened by the action potential is it unable to reduce cellular calcium concentration. As the action potential decreases, then the calcium gates close and the sarcoplasmic reticulum “catches up” and cellular calcium concentrations fall. At that point troponin releases calcium, tropomyosin goes back to covering myosin binding sites on actin, myosin loses its attachment to actin and the thin filaments slide back to their original positions relative to the myosin thick filaments. Figure 2.123 - The Sliding Filament Model of Muscular Contraction Wikipedia Tropomyosin Figure 2.124 - Tropomyosin and troponin in muscle anatomy Wikipedia Tropomyosins are proteins that interact with actin thin filaments to help regulate their roles in movement, both in muscle cells and non-muscle cells (Figure 2.124). Tropomyosins interact to form head-to-toe dimers and perch along the α-helical groove of an actin filament. The isoforms of tropomyosin that are in muscle cells control interactions between myosin and the actin filament within the sarcomere and help to regulate contraction of the muscle. In other cells, nonmuscle tropomyosins help to regulate the cytoskeleton’s functions. The interactions of tropomyosin with the cytoskeleton are considerably more complicated than what occurs in muscle cells. Muscle cells have five tropomyosin isoforms, but in the cytoskeleton of non-muscle cells, there are over 40 tropomyosins. Troponin Figure 2.125 - Troponin complex of muscle. Blue = troponin C, magenta = troponin T , green = troponin I The troponins involved in muscular contraction are actually a complex of three proteins known as troponin I, troponin C, and troponin T (Figure 2.125). They associate with each other and with tropomyosin on actin filaments to help regulate the process of muscular contraction. Troponin I prevents binding of myosin’s head to actin and thus prevents the most important step in contraction. Troponin C is a unit that binds to calcium ions. Troponin T is responsible for binding all three proteins to tropomyosin. Troponins in the bloodstream are indicative of heart disorders. Elevation of troponins in the blood occurs after a myocardial infarction and can remain high for up to two weeks. Actinin Actinin is a skeletal muscle protein that attaches filaments of actin to Z-lines of skeletal muscle cells. In smooth muscle cells, it also connects actin to dense bodies. Titin Titin (also known as connectin) is the molecular equivalent of a spring that provides striated muscle cells with elasticity. It is the third most abundant protein in muscle cells. The protein is enormous, with 244 folded individual protein domains spread across 363 exons (largest known number), with the largest known exon (17,106 base pairs long), and it is the largest protein known (27,000 to 33,000 amino acids, depending on splicing). Unstructured sequences The folded protein domains are linked together by unstructured sequences. The unstructured regions of the protein allow for unfolding when stretching occurs and refolding upon relaxation. Titin connects the M and Z lines in the sarcomere (Figure 2.123). Tension created in titin serves to limit the range of motion of the sarcomere, giving rise to what is called passive stiffness. Skeletal and cardiac muscles have slight amino acid sequence variations in their ti tin proteins and these appear to relate to differences in the mechanical characteristics of each muscle. Energy backup for muscle energy Myoglobin was described as a molecular batter for oxygen. Muscle cells have a better of their own for ATP. The is important for animals, but not for plants because a plant’s need for energy is different than an animal’s. Plants do not need to access energy sources as rapidly as animals do, nor do they have to maintain a constant internal temperature. Plants can neither flee predators, nor chase prey. These needs of animals are much more immediate and require that energy stores be accessible on demand. Muscles, of course, enable the motion of animals and the energy required for muscle contraction is ATP. To have stores of energy readily available, muscles have, in addition to ATP, creatine phosphate for energy and glycogen for quick release of glucose to make more energy. The synthesis of creatine phosphate is a prime example of the effects of concentration on the synthesis of high energy molecules. For example, creatine phosphate has an energy of hydrolysis of -43.1 kJ/mol whereas ATP has an energy of hydrolysis of -30.5 kJ/mol. Creatine phosphate, however, is made from creatine and ATP in the reaction shown in Figure 2.126. How is this possible? Figure 2.126 - Phosphorylation of creatine (phosphocreatine) - making of a creatine phosphate battery Image by Aleia Kim The ∆G°’ of this reaction is +12.6 kJ/mol, reflecting the energies noted above. In a resting muscle cell, ATP is abundant and ADP is low, driving the reaction downward, creating creatine phosphate. When muscular contraction commences, ATP levels fall and ADP levels climb. The above reaction then reverses and proceeds to synthesize ATP immediately. Thus, creatine phosphate acts like a battery, storing energy when ATP levels are high and releasing it almost instantaneously to create ATP when its levels fall.