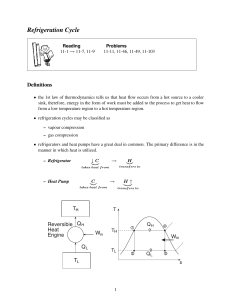
Introduction How does it work? High Temperature Reservoir Heat Rejected R Work Input Heat Absorbed Low Temperature Reservoir A N Khudaiwala,G.P.Porbandar Refrigeration It is defined as the process of providing and maintaining a temperature well below that of surrounding atmosphere. In other words refrigeration is the process of cooling substance. A N Khudaiwala,G.P.Porbandar Refrigerators and heat pumps If the main purpose of the machine is to cool some object the machine is named as refrigerator If the main purpose of machine to heat a medium warmer than the surroundings, the machine is termed as heat pump. A N Khudaiwala,G.P.Porbandar Refrigerator and Heat pump Warm Space QR Work Input Heat Pum p QR Work Input Refri gerat or Cold Space A N Khudaiwala,G.P.Porbandar Refrigeration REFRIGERATION – Science of producing and maintaining temperature below that of REFRIGERATION – Cooling of or removal of heat from a system. surrounding / atmosphere. Refrigerating System – Equipment employed to maintain the system at a lowtemperature. Refrigerated System – System which is kept at lower temperature. Refrigeration – 1) By melting of a solid, 2) By sublimation of a solid, 3) By evaporation of a liquid. Most of the commercial refrigeration production : Evaporation of liquid. This liquid is known as Refrigerant. ME0223 SEM-IV Applied Thermodynamics & Heat Engines A N Khudaiwala,G.P.Porbandar Refrigerating EffectDefinitions (N): It is defined as the quantity of heat extracted from a cold body or space to be cooled in a given time. N= Heat extracted from the cold space Time taken Specific Heat of water and ice : It is the quantity of heat required to raise or lower the temperature of one kg of water (or ice), through one kelvin or (10 c) in one second. Specific heat of water, Cpw = 4.19 kJ/kg K Specific heat of ice, Cpice = 2.1 kJ/kg K. A N Khudaiwala,G.P.Porbandar Definitions Capacity of a Refrigeration Unit : Capacity of a refrigerating machines are expressed by their cooling capacity. The standard unit used for expressing the capacity of refrigerating machine is ton of refrigeration. One ton of refrigeration is defined as, “the quantity of heat abstracted (refrigerating effect) to freeze (into ice) one ton of water in a duration of 24 hours at 0o c”. Heat extracted from at oo c = latent heat of ice Latent heat of ice = 336 kJ/kg i.e., 336 kJ of heat should be extracted one kg of water at 0o C to convert it into ice. A N Khudaiwala,G.P.Porbandar One ton of Refrigeration One ton of refrigeration= 336x1000 kJ/24 hrs. = 336x1000 kJ/min 24x60 One ton of refrigeration = 233.333 kJ/min = 3.8889 kJ/sec For calculation purpose, One ton of refrigeration = 12600 kJ/hr = 210 kJ/min Ton of refrigeration = 3.5 kJ/s A N Khudaiwala,G.P.Porbandar - COP Performance of Definitions Refrigerators (Co efficient of Refrigerators) : The performance of heat engine is expressed by its thermal efficiency. The performance of a refrigerator cannot be expressed in terms of efficiency. In case of a refrigerator the aim is to extract maximum quantity of heat from the sink with minimum of work input. Hence a new term Co efficient of Performance is brought into use to express the performance of refrigerator. A N Khudaiwala,G.P.Porbandar Definitions -ItCOP Co efficient of Performance: is defined as the ratio of heat extracted in a given time (refrigerating effect) to the work input. Co efficient of performance = Heat extracted in evaporator Work Input Co efficient of performance = Refrigerating Effect Work Input Co efficient of performance = N W The COP is always greater than 1 and known as theoretical coefficient of performance. A N Khudaiwala,G.P.Porbandar Performance - Rating Rating of Refrigeration System : - Refrigeration Effect / Amount of Heat extracted from a body in a given time. Definition : - Refrigeration Effect produced by melting 1 tonne of ice from and at 0 ºC in 24 hours. Unit : - Standard commercial Tonne of Refrigeration / TR Capacity Latent Heat of ice = 336 kJ/kg. A N Khudaiwala,G.P.Porbandar Air Refrigeration System One of the earliest method. Obsolete due to low COP and high operating cost. Preferred in Aircraft Refrigeration due to its low weight. Characteristic : - Throughout the cycle, Refrigerant remains in gaseous state. Air Refrigeration Closed System • Air refrigerant contained within piping or components of system. • Pressures above atm. Pr. Open System • Refrigerator space is actual room to be cooled. • Air expansion to atm. Pr. And then compressed to cooler pressure. A N Khudaiwala,G.P.Porbandar • Pressures limited to near atm. Pr. levels.. Air Refrigeration System Closed System Vs. Open System : 1. Suction to compressor in Closed System may be at high pressures. Hence, the size of Expander and Compressor can be kept small. 2. In Open Systems, air picks up the moisture from refrigeration chamber. This moisture freezes and chokes the valves. 3. Expansion in Open System is limited to atm. Pr. Level only. No such restriction to Closed System. A N Khudaiwala,G.P.Porbandar Isotherms 3 T1 2 Adiabatic Expansion Temperature Pressure Reverse Carnot Cycle Compression 4 T2 3 2 Expansion T1 Compression 4 1 4’ 1’ 1 T2 Entropy Volume P –V Diagram A N Khudaiwala,G.P.Porbandar T –s Diagram Reverse Carnot Cycle Temperature Operation : T2 3 1 – 2 : Adiabatic Compression. Requires external power. 2 Temp. rises from T1 to T2. Expansion T1 Cylinder in contact with Hot Body at T2 Compression 1 4 2 – 3 : Isothermal Compression. Heat Rejection to Hot Body. 3 – 4 : Adiabatic Expansion. 4’ Temp. falls from T2 to T1. 1’ Cylinder in contact with Cold Body at T1. Entropy 4 – 1 : Isothermal Expansion. Heat Extraction from Cold Body. A N Khudaiwala,G.P.Porbandar Reverse Carnot Cycle Heat extracted from cold Body : Area 1-1’-4’-4 Temperature = T1 X 1-4 T2 3 2 Expansion T1 Work done per cycle = (T2 – T1) X 1-4 Compression 4 1 4’ 1’ : Area 1-2-3-4 Heat Extracted Work Done Area 1 − 1'−4'−4 = Area 1 − 2 − 3 − 4 COP = = T1 X (1 − 4) (T2 − T1 ) X (1 − 4) = T1 T2 − T1 Entropy A N Khudaiwala,G.P.Porbandar Example 1 A Carnot Refrigerator requires 1.3 kW per tonne of refrigeration to maintain a region at low temperature of -38 ºC. Determine: i)COP of Carnot Refrigerator. ii)Higher temperature of the cycle. iii)Heat delivered and COP, if the same device is used Heat Pump. Heat absorbed 1 tonne 14,000 kJ / hr COPrefrig = = = = 2.99….ANS Work done 1.3 kW (1.3 kW ) (3600 sec/ hr ) T1 235 K COPrefrig = ⇒ 2.99 = ⇒ T1 = 313.6 K ….ANS T2 − T1 T2 − 235 K Heat Delivered as Heat Pump ; = Heat absorbed + Work done 14,000 kJ / hr + 1.3 = 5.189 kJ / sec ….ANS 3600 Heat delivered 5.189 kJ / sec COPHP = = = 3.99 ….ANS Work done 1.3 kW = 1 tonne + 1.3 kW = A N Khudaiwala,G.P.Porbandar Example 2 A refrigerating system works on reverse Carnot cycle. The higher temperature in the system is 35 ºC and the lower temperature is -15 ºC. The capacity is to be 12 tonnes. Determine : i)COP of Carnot Refrigerator. ii)Heat rejected from the system per hour. iii)Power required. COPrefrig = T1 258 K = = 5.18 ….ANS T2 − T1 308 K − 258 K Re frig . Effect 12 tonne 12 X 14,000 kJ / hr ⇒ 5.16 = = Work Input Work Input Work Input ⇒ Work Input = 32558 kJ / hr COPrefrig = Heat Rejected / hr = Refrig. Effect / hr + Work Input / hr = 12 x 14,000 (kJ/hr) + 32,558 (kJ/hr) = 2,00,558 kJ/hr.….ANS Power = Work Input / hr 32558 kJ / hr = = 9.04 kW ….ANS 3600 3600 A N Khudaiwala,G.P.Porbandar Example 3 Ice is formed at 0 ºC from water at 20 ºC. The temperature of the brine is -8 ºC. Find out the kg of ice per kWh. Assume that the system operates on reversed Carnot cycle. Take latent heat of ice as 335 kJ/kg. COPrefrig T1 265 K = = = 9.46 T2 − T1 293 K − 265 K Heat to be extracted per kg of water ( to from ice at 0 ºC) Rn = 1 (kg) x Cpw (kJ/kg.K) x (293– 273) (K) + Latent Heat (kJ/kg) of ice = 1 (kg) x 4.18 (kJ/kg.K) x 20 (K) + 335 (kJ/kg) = 418.6 kJ/kg. Also, 1 kWh = 1 (kJ) x 3600 (sec/hr) = 3600 kJ. Rn Re frig . Effect (kJ ) = W Work done (kJ ) m (kg ) X 418.6 (kJ / kg ) ⇒ 9.46 = ice ⇒ mice = 81.35 kg….ANS 3600 kJ COPrefrig = A N Khudaiwala,G.P.Porbandar Bell – Coleman / Reverse Bryaton Cycle Elements of this system : Cooling Water Heat Exchanger Cold Air 1. Compressor. Hot Air 2. Heat Exchanger. 3. Expander. Expander Compressor Very Cold Air 4. Refrigerator. Warm Air Work gained from Expander is used to drive Compressor. Refrigerator Hence, less external work is required. A N Khudaiwala,G.P.Porbandar Isobars Isobars 2 2 3 Adiabatic Expansion Compression 1 4 Temperature Pressure Bell – Coleman / Reverse Bryaton Cycle 3 Compression Expansion 4 Entropy Volume P –V Diagram A N Khudaiwala,G.P.Porbandar Adiabatic T –s Diagram 1 Bell – Coleman / Reverse Bryaton Cycle Heat Absorbed in Refrigerator : Qadded = m C P (T1 − T4 ) Heat Rejected in Heat Exchanger : Isobars Temperature 2 3 Adiabatic Qrejected = m C P (T2 − T3 ) If process changes from Adiabatic to Polytropic; Compression Expansion 4 1 Qexp n Entropy n ( P2 V2 − P1 V1 ) n −1 n ( P3 V3 − P4 V4 ) = n −1 Qcomp = We know, A N Khudaiwala,G.P.Porbandar γ −1 R = C P γ Bell – Coleman / Reverse Bryaton Cycle Net Work Done : W = Wcomp − Wexp n n ( P2 V2 − P1 V1 − P3 V3 + P4 V4 ) = n −1 n = m R ( T2 − T1 − T3 + T4 ) n −1 n γ −1 m C P ( T4 − T3 + T2 − T1 ) = n −1 γ For Isentropic Process : W = Wcomp − Wexp n = m C P ( T4 − T3 + T2 − T1 ) A N Khudaiwala,G.P.Porbandar Bell – Coleman / Reverse Bryaton Cycle COP : Qadded Work Added COP = = Qrejected − Qadded Wnet = m C P (T1 − T4 ) n γ −1 m C P ( T4 − T3 + T2 − T1 ) n −1 γ COP = (T1 − T4 ) n γ −1 ( T4 − T3 + T2 − T1 ) n −1 γ A N Khudaiwala,G.P.Porbandar Example 4 A Bell – Coleman refrigerator operates between pressure limits of 1 bar and 8 bar. Air is drawn from the cold chamber at 9 ºC, compressed and then cooled to 29 ºC before entering the expansion cylinder. Expansion and compression follow the law PV1.35 = Const. Calculate the theoretical COP. For air, take γ = 1.4 and Cp = 1.003 kJ/kg. Polytropic Compression 1-2 : 2 Pressure 302 K P2 = 8 bar 3 P1 = 1 bar P2 T2 = T1 P1 n −1 n 8 bar = (282 K ) 1 bar 1.35−1 1.35 = 482.2 K PV1.35=C Polytropic Expansion 3-4 : 1 282 K 4 Volume n −1 n P3 T3 = T4 ⇒ (302 K ) = T4 P4 ⇒ T4 = 176.6 K A N Khudaiwala,G.P.Porbandar 8 bar 1 bar 1.35 −1 1.35 Example 4….contd Heat Extracted from Cold Chamber : = C P (T1 − T4 ) = 1.003 (kJ / kg ) X (282 K − 176.6 K ) = 105.7 kJ / kg Heat Rejected to Heat Exchanger : = C P (T2 − T3 ) = 1.003 (kJ / kg ) X (482.2 K − 302 K ) = 180.7 kJ / kg Net Work Done : Wnet Wnet Wnet n γ −1 m C P ( T4 − T3 + T2 − T1 ) = n −1 γ 1.35 1.4 − 1 = (1.003 kJ / kg ) (176.6 K − 302 K + 482.2 K − 282 K ) 1.35 − 1 1.4 = 82.8 kJ / kg COPrefrig Heat absorbed 105.7 kJ / kg = = = 1.27….ANS Work done 82.8 kJ / kg A N Khudaiwala,G.P.Porbandar Example 5 An air refrigeration open system operating between 1 MPa and 100 kPa is required to produce a cooling effect of 2000 kJ/min. temperature of the air leaving the cold chamber is -5 ºC, and at leaving the cooler is 30 ºC. Neglect losses and clearance in the compressor and expander. Determine : i)Mass of air circulated per min. ii) Compressor Work, Expander Work, Cycle Work. ii)COP and Power in kW required. Polytropic Expansion 3-4 : 2 Pressure 303 K P2 3 = 1 MPa γ −1 γ P1 = 100 kPa PVγ=C P T3 = T4 3 ⇒ (302 K ) = T4 P4 ⇒ T4 = 156.9 K 1 MPa 0.1 MPa Refrig. Effect per kg : 1 268 K 4 Volume = C P (T1 − T4 ) = 1.003 (kJ / kg ) X (268 K − 156.9 K ) = 111.66 kJ / kg A N Khudaiwala,G.P.Porbandar 1.4 −1 1 .4 Example 5….contd Mass of air circulated per min : Re frig . Effect 2000 kJ / min = = = 17.91 kg / min ….ANS Re frig . Effect per kg 111.66 kJ / kg P2 Polytropic Compression 1-2 :T2 = T1 P1 γ −1 γ 1000 kPa = (268 K ) 100 kPa 1.4 −1 1.4 = 517.4 K Compressor Work : γ m R ( T2 − T1 ) Wcomp = γ −1 1.4 Wcomp = (17.91 kg / min) (0.287 kJ / kg ) ( 517.4 K − 268 K ) 1.4 − 1 Wcomp = 4486.85 kJ / min ….ANS A N Khudaiwala,G.P.Porbandar ….ANS Example 5….contd Expander Work : γ m R ( T3 − T4 ) Wexp = γ −1 1.4 Wcomp = (17.91 kg / min) (0.287 kJ / kg ) ( 303 K − 156.9 K ) 1.4 − 1 Wcomp = 2628.42 kJ / min….ANS cle Work = Wcycle = Wcomp – Wexp = 4486.85 kJ/min – 2628.42 kJ/min COPrefrig = 1858.43 kJ/min…ANS Re frig . Effect 2000 kJ / min = = = 1.076 ….ANS Work required 1858.43 kJ / min Wcycle 1858.43 kJ / min = = 30.97 kW….ANS Power required : P = time 60 sec/ min A N Khudaiwala,G.P.Porbandar Air Refrigeration Cycle - Merits / Demerits Merits : 1. No risk of fire (as in case of NH3); as air is non – flammable. 2. Cheaper (than other systems); as air is easily available. 3. Weight per tonne of refrigeration is quite low (compared to other systems). Demerits : 1. Low COP (compared with other systems). 2. Weight of air (as Refrigerant) is more (compared to other systems). A N Khudaiwala,G.P.Porbandar Refrigeration - Applications 1. Ice making. 2. Transportation of food items above and below freezing. 2. Industrial Air – Conditioning. 4. Comfort Air – Conditioning. 5. Chemical and related industries. 6. Medical and Surgical instruments. Applications : 7. Processing food products and beverages. 8. Oil Refining. 9. Synthetic Rubber Manufacturing. 10. Manufacture and treatment of metals. 11. Freezing food products. 12. Manufacturing Solid Carbon Dioxide. 13. Production of extremely low temperatures (Cryogenics) 14. Plumbing. 15. Building Construction. A N Khudaiwala,G.P.Porbandar Applications of Refrigeration In chemical industries, for separating and liquefying the gases. In manufacturing and storing ice. For the preservation of perishable food items in cold storages. For cooling water. For controlling humidity of air manufacture and heat treatment of steels. For chilling the oil to remove wax in oil refineries. For the preservation of tablets and medicines in pharmaceutical industries. For the preservation of blood tissues etc., For comfort air conditioning the hospitals, theatres, etc., A N Khudaiwala,G.P.Porbandar Types of Refrigerators Ice Refrigerators : Ice is kept in the cabinet of refrigerators and this acts as the refrigerating means. Air Refrigerators : Air is used as working agent in these types of refrigerators. E.g., Bell Coleman Cycle. Vapour Refrigerators: The working agents employed in this type of refrigerators are ammonia, CO2, SO2, freons etc., A N Khudaiwala,G.P.Porbandar Vapour Compression Refrigeration System A N Khudaiwala,G.P.Porbandar Refrigeration - Elements High Temp Source Surrounding Air QH Condenser QH Wnet, in Wnet, in Expansion Valve Compressor Evaporator QL QL Refrigerated Space A N Khudaiwala,G.P.Porbandar Low Temp Sink Vapour Compression Refrigeration System Construction This system consists of a compressor, condenser, a receiver tank, an expansion valve and an evaporator. Compressor : Reciprocating compressors generally used. For very big plants centrifugal compressors directly coupled with high speed rotating engines (gas turbine) are used. A N Khudaiwala,G.P.Porbandar Vapour Compression Refrigeration System Construction Compressor: For very big plants Centrifugal compressors directly coupled with high speed rotating engines (gas turbine) are used A N Khudaiwala,G.P.Porbandar Vapour Compression Refrigeration System Construction Condenser : It is a coil of tubes made of copper. Receiver tank: It is the reservoir of liquid refrigerant. Expansion Valve: This is a throttle valve. High pressure refrigerant is made to flow at a controlled rate through this valve. Evaporator : It is the actual cooler and kept in the space to be cooled. The evaporator is a coil of tubes made of copper A N Khudaiwala,G.P.Porbandar Vapour Compression Refrigeration System Working Working : 1. The low pressure refrigerant vapour coming out of the evaporator flows into the compressor. 2. The compressor is driven by a prime mover. 3. In the compressor the refrigerant vapour is compressed. 4. The high pressure refrigerant vapour from the compressor is then passed through the condenser. 5. The refrigerant gives out the heat it had taken in the evaporator (N) A N Khudaiwala,G.P.Porbandar Vapour Compression Refrigeration System Working Working : 6. The heat equivalent of work done on it (w) on the compressor. 7. This heat is carried by condenser medium which may be air or water. 8. The high pressure liquid refrigerant then enters the expansion valve. 9. This valve allows the high pressure liquid refrigerant to flow at a controlled rate into the evaporator. 10. While passing though this valve the liquid partially evaporates. A N Khudaiwala,G.P.Porbandar Vapour Compression Refrigeration System Working Working : 11.Most of the refrigerant is vapourised only in the evaporator, at a low pressure. 12. In the evaporator the liquid refrigerant absorbs its latent heat of vapourisation from the material which is to be cooled. 13. Thus the refrigerating effect (N) is obtained. 14. Then the low pressure refrigerant enters the Khudaiwala,G.P.Porbandar compressorA Nand the cycle is repeated. Refrigeration Circuit Evaporator Compressor Refrigeration Circuit Expansion Valve ME0223 SEM-IV Condenser A N Khudaiwala,G.P.Porbandar Applied Thermodynamics & Heat Engines Vapour Compression System Elements of this system : 3 1. Compressor. 4 2. Condenser. 2 1 3 2 3. Expansion Valve. 4. Evaporator. Vapour @ ↓ Pr. and ↓ Temp. (State 1) Isentropic Compression : ↑ Pr. and ↑ Temp. (State 2) Condenser : ↑ Pr. Liquid (State 3) 4 1 Throttling : ↓ Pr. ↓ Temp. (State 4) Evaporator : Heat Extraction from surrounding; ↓ Pr. vapour (State 1). A N Khudaiwala,G.P.Porbandar Vapour Compression System Merits : 1. High COP; as very close to Reverse Carnot Cycle. 2. Running Cost is 1/5th of that of Air Refrigeration Cycle. 3. Size of Evaporator is small; for same Refrigeration Effect. 4. Evaporator temperature adjustment is simple; by adjusting Throttle Valve. Demerits : 1. Initial cost is high. 2. Inflammability. 3. Leakage. 4. Toxicity. A N Khudaiwala,G.P.Porbandar Vapour Compression System : T-s Diagram Case A. Dry and Saturated Vapour after Compression : Temperature, T Work done by Compressor = W = Area 1-2-3-4-1 T2 Compressor Work, Condensation2 (W) 3 Heat Absorbed = W = Area 1-4-g-f-1 Sat. Vapour Line Expansion T1 4 g Sat. Liq. Line Evaporation Heat Absorbed COP = Compression Work Done 1 Area 1 − 4 − g − f − 1 = Net Refrig. Effect, Area 1 − 2 − 3 − 4 − 1 (R ) f n Entropy, s A N Khudaiwala,G.P.Porbandar = h1 − h4 h2 − h1 Vapour Compression System : T-s Diagram Case B. Superheated Vapour after Compression : Temperature, T 2 T2 3 Condensation 2’ Compressor Work, (W) Sat. Vapour Line Expansion T1 4 g Sat. Liq. Line Compression 1 Evaporation f Net Refrig. Effect, (Rn) Entropy, s Work done by Compressor = W = Area 1-2-2’-3-4-1 Heat Absorbed = W = Area 1-4-g-f-1 Heat Absorbed Work Done Area 1 − 4 − g − f − 1 = Area 1 − 2 − 2'−3 − 4 − 1 COP = h1 − h4 = h2 − h1 NOTE : h2 = h2’ + Cp (Tsup – Tsat) A N Khudaiwala,G.P.Porbandar Vapour Compression System : T-s Diagram Case C. Wet Vapour after Compression : Temperature, T Work done by Compressor = W = Area 1-2-3-4-1 3 Condensation T2 Compressor Work, (W) 2 Sat. Vapour Line Expansion T1 4 g Sat. Liq. Line Compression Evaporation f 1 Net Refrig. Effect, (Rn) Entropy, s Heat Absorbed = W = Area 1-4-g-f-1 Heat Absorbed Work Done Area 1 − 4 − g − f − 1 = Area 1 − 2 − 3 − 4 − 1 COP = h1 − h4 = h2 − h1 NOTE : h2 = (hf + x.hfg)2 A N Khudaiwala,G.P.Porbandar Vapour Compression System : P-h Diagram Isobaric, P = Const S ent = rop C o ic ns , t. . Line Is 2 – phase region Sat. Va p Isenthalpic, h = Const. Const. D ryness Fraction Sub-cooled Liq. region Sat. Liq. L ine Pressure, Pr Isothermal, T = Const , ric . o ch nst I so Co Superheated v= region Enthalpy, h A N Khudaiwala,G.P.Porbandar Condensation s io n 2 Evaporation Co mp res 3 Compression Pressure, Pr Vapour Compression System : P-h Diagram 1 4 Enthalpy, h Rn = h1 − h4 W = h2 − h1 } Rn h1 − h4 COP = = W h2 − h1 A N Khudaiwala,G.P.Porbandar Factors Affecting Vapour Compression System A. Effect of Suction Pressure : Pressure, Pr COP of Original Cycle : P2 P1 2 2’ 3 4 4’ Rn h1 − h4 COP = = W h2 − h1 COP when Suction Pr. decreased : Rn h1' − h4 ' COP = = W h2 ' − h1' 1 = 1’ ( h1 − h4 ) − ( h1 − h1' ) ( h2 − h1 ) + ( h1 − h1' ) + ( h2' − h2 ) Thus, Refrig. Effect ↓ Enthalpy, h Work Input ↑ ⇒ COP ↓ A N Khudaiwala,G.P.Porbandar Factors Affecting Vapour Compression System B. Effect of Delivery Pressure : Pressure, Pr COP of Original Cycle : P2 3 3’ 2’ 2 P1 4 Rn h1 − h4 COP = = W h2 − h1 COP when Delivery Pr. increased : Rn h1 − h4 ' COP = = W h2 ' − h1 1 = 4’ ( h1 − h4 ) − ( h4' − h4 ) ( h2 − h1 ) + ( h2' − h2 ) Thus, Refrig. Effect ↓ Enthalpy, h Work Input ↑ ⇒ COP ↓ A N Khudaiwala,G.P.Porbandar Factors Affecting Vapour Compression System C. Effect of Superheating : Pressure, Pr COP of Original Cycle : P2 P1 2 3 4 2’ Rn h1 − h4 COP = = W h2 − h1 COP when Delivery Pr. increased : Rn h1' − h4 COP = = W h2 ' − h1' 1 = 1’ ( h1 − h4 ) + ( h1' − h1 ) ( h2 − h1 ) + ( h2' − h2 ) + ( h1' − h1 ) Thus, Refrig. Effect ↑ Work Input ↑ Enthalpy, h ⇒ A N Khudaiwala,G.P.Porbandar or ↓ COP ↓ or ↑ Factors Affecting Vapour Compression System E. Effect of Suction & Condenser Temperatures : Temperature, T COP of Original Cycle : Heat Absorbed COP = Work Done T2 3 2’ 3’ 4 Compression Evaporation g 1 COP of Modified Cycle : Heat Absorbed ( ↑ ) Work Done ( ↓ ) Area 1 − 1'−4 − 4'− g − f − 1 = Area 1'−2'−3'−4'−1' COP = f Entropy, s ⇒ A N Khudaiwala,G.P.Porbandar h1 − h4 h2 − h1 Evaporator Temp. ↑ 1’ 4’ = Condenser Temp. ↓ Now, Expansion T1 Area 1 − 4 − g − f − 1 Area 1 − 2 − 3 − 4 − 1 = Condensation2 COP ↑ > h1 − h4 h2 − h1 Vapour Compression System – Mathematical Analysis A. Refrigerating Effect : = Amount of Heat absorbed in Evaporator. Qevap = ( h1 − h4 ) + Latent Heat + Superheated Heat (kJ / kg ) B. Mass of Refrigerant : = Amount of Heat absorbed / Refrigerating Effect. m= 14,000 3600 ( h1 − h4 ) ( kg / sec− tonne) 1 TR = 14,000 kJ/hr C. Theoretical Piston Displacement : = Mass of Refrigerant X Sp. Vol. of Refrigerant Gas (vg)1. 14,000 Th. Piston Displ. = ∗ ( v g )1 (m 3 / sec− tonne) 3600 ( h1 − h4 ) ME0223 SEM-IV A N Khudaiwala,G.P.Porbandar Applied Thermodynamics & Heat Engines Vapour Compression System – Mathematical Analysis D. Theoretical Power Required : a) Isentropic Compression : Wcomp = ( h2 − h1 ) Ptheor = m ( h2 − h1 ) (kJ / kg ) (kW ) a) Polytropic Compression : n ( P2V2 − P1V1 ) (kJ / kg ) Wcomp = n −1 n ( P2V2 − P1V1 ) (kW ) Ptheor = m n −1 E. Heat removed through Condenser : Qcond = m ( h2 − h3 ) A N Khudaiwala,G.P.Porbandar ( kJ / kg ) Example 6 7 A refrigeration machine is required to produce ice at 0º C from water at 20 ºC. The machine has a condenser temperature of 298 K while the evaporator temperature is 268 K. The relative efficiency of the machine is 50 % and 6 kg of Freon-12 refrigerant is circulated through the system per minute. The refrigerant enters the compressor with a dryness fraction of 0.6. Specific heat of water is 4.187 kJ/kg.K and the latent heat of ice is 335 kJ/kg. Calculate the amount of ice produced on 24 hours. The table of properties if Freon-12 is given below: Given : } Temperature (K) Liquid Heat (kJ/kg) Latent Heat (kJ/kg) Entropy of Liquid (kJ/kg) 298 59.7 138.0 0.2232 268 31.4 154.0 0.1251 m = 6 kg/min hf1 = 31.4 kJ/kg ηrel = 50 % hfg1 = 154.0 kJ/kg x2 = 0.6 hf2 = 59.7 kJ/kg Cpw = 4.187 kJ/kg.K hfg2 = 138.0 kJ/kg Latent Heat of ice = 335.7 kJ/kg hf3 = h4 = 59.7 kJ/kg A N Khudaiwala,G.P.Porbandar Example 6….contd h1 = h f1 + x h fg1 = 31.4 + (0.6)154.0 = 123.8 kJ / kg Temperature, T Isentropic Compression : 1-2 298 K 3 s2 = s1 2 s f 2 + x2 ∗ s fg 2 = s f 1 + x1 ∗ s fg1 h2 = h f 2 + x2 h fg 2 = 59.7 + (0.5325)138.0 = 133.2 kJ / kg h fg 2 h fg1 = s f 1 + x1 s f 2 + x2 T2 T1 138.0 154.0 ( ) 0.2232 + x2 = 0 . 1251 + 0 . 6 298 268 ⇒ x2 = 0.5325 Sat. Vapour Line 268 K 4 g Sat. Liq. Line 1 f Entropy, s h4 = h f 3 = 59.7 kJ / kg ( Rn h1 − h4 123.8 − 59.7 ) kJ / kg = = = 6.82 COP of Original Cycle : COP = W h2 − h1 (133.2 − 123.8) kJ / kg ME0223 SEM-IV A N Khudaiwala,G.P.Porbandar Applied Thermodynamics & Heat Engines Example 6….contd Actual COP = ηrel X COPtheor = 0.5 X 6.82 = 3.41 Temperature, T Heat extracted from 1 kg of water at 20 ºC to form 1 kg of ice at 0 ºC : 298 K 3 = 1 (kg ) X 4.187 (kJ / kg.K ) X (20 − 0) (°C ) + 335 (kJ / kg ) = 418.74 kJ / kg 2 Sat. Vapour Line 268 K 4 g Sat. Liq. Line 1 f Entropy, s A N Khudaiwala,G.P.Porbandar Now; COPactual = 3.41 = Rn ( actual ) W mice X 418.74 = m ( h2 − h1 ) 6 (kg ) X (133.2 − 123.8) (kJ / kg ) ⇒ mice = ∗ 3.41 418.74 kJ / kg = 0.459 kg / min 0.459 X 60 X 24 = = 0.661 tonne in 24 hrs 1000 ….ANS Example 7 28 tonnes of ice from and at 0 ºC is produced per day in an ammonia refrigerator. The temperature range in the compressor is from 25 ºC to -15oC. The vapour is dry and saturated at the end of compression and an expansion valve is used. Assuming a co-efficient of performance of 62% of the theoretical, calculate the power required Temp Enthalpy (kJ/kg) Entropy of Entropy of Vapour to (ºC) Liquid (kJ/kg.K) Liquid Vapour drive the compressor. Take latent heat of ice = 335 kJ/kg. (kJ/kg.K) Given : } 25 100.04 1319.22 0.3473 4.4852 -15 -54.56 1304.99 -2.1338 5.0585 Tcond = 25 ºC hf1 = -54.56 kJ/kg Tevap = -15 ºC hg1 = 1304.99kJ/kg x2 = 1….dry saturated vapour hf2 = 100.04 kJ/kg COPactual = 0.62 (COPtheor) hg2 = 1319.22 kJ/kg Latent Heat A N Khudaiwala,G.P.Porbandar of ice = 335.7 kJ/kg hf3 = h4 = 100.04 kJ/kg Example 7….contd h2 = hg 2 = 1319.22 kJ / kg h3 = h4 = 100.04 kJ / kg.....Isenthalpic process Isentropic Compression : 1-2 Temperature, T s2 = s1 298 K 3 s g 2 = s f 1 + x1 ∗ s fg1 2 Sat. Vapour Line 258 K ⇒ 4 g Sat. Liq. Line 4.4852 = (−2.1338) + ( x1 ) [ 5.0585 − ( − 2.1338) ] 1 x2 = 0.92 h1 = h f 1 + x1 (h fg1 ) = (−54.56) + (0.92) [1304.99 − (−54.56)] f = 1196.23 kJ / kg Entropy, s COP of the Cycle : COPtheoretical = (1196.23 − 100.04) = 8.91 h1 − h4 = h2 − h1 (1319.22 − 1196.23) A N Khudaiwala,G.P.Porbandar Example 7….contd Actual Rn = COPactual X Work done Temperature, T Actual COP = ηrel X COPtheor = 0.62 X 8.91 = 5.52 X (h2 – h1) = 5.52 = 5.52 X (1319.22 – 1196.23) 298 K 3 2 = 678.9 kJ/kg Heat extracted from 28 tonnes of water at 0 ºC to form ice at 0 ºC : Sat. Vapour Line 258 K 4 g Sat. Liq. Line 1 28 (kg ) X 1000 (kg / tonne) X 335 (kJ / kg ) = 24 (hr ) X 3600 (sec/ hr ) = 108.56 kJ / sec (kW ) Mass of refrigerant : = f Entropy, s Total Work done by Compressor : 108.56 (kJ / sec) = 0.1599 kg 678.9 (kJ / kg ) = mrefrig X ( h2 − h1 ) = 0.1599 (kg ) X (1319.22 − 1196.23) kJ / kg = 19.67 kJ / sec (kW ) ….ANS A N Khudaiwala,G.P.Porbandar Example 8 In a standard vapour compression refrigeration cycle, operating between an evaporator temperature of -10 ºC and a condenser temperature of 40 ºC, the enthalpy of the refrigerant, Freon-12, at the end of compression is 220 kJ/kg. Show the cycle diagram on T-s plane. Calculate: 1. The C.O.P. of the cycle. 2. The refrigerating capacity and the compressor power assuming a refrigerant flow rate Temp of 1 kg/min. (ºC) Pr (MPa) hf (kJ/kg) hg (kJ/kg) You may use the extract of Freon-12 property table given below: Given : } -10 0.2191 26.85 183.1 40 0.9607 74.53 203.1 Tcond = 40 ºC Tevap = -10 ºC x1 = 1….dry saturated vapour h2 = 220 kJ/kg A N Khudaiwala,G.P.Porbandar hf1 = 26.85 kJ/kg hg1 = h1 = 183.1 kJ/kg hf2 = 74.53 kJ/kg hg2 = 203.1 kJ/kg hf3 = h4 = 74.53 kJ/kg Example 8….contd COP of Original Cycle : COP = Temperature, T = 2 40 ºC 3 Rn h1 − h4 = W h2 − h1 (183.1 − 74.53) kJ / kg = 2.94 ….ANS ( 220.0 − 183.1) kJ / kg 2’ Refrigerating Capacity : = m ( h1 − h4 ) = 1 (kg ) X (183.1 − 74.53) kJ / kg -10 ºC 1 4 Sat. Vapour Line g Sat. Liq. Line = 108.57 kJ / min ….ANS f Entropy, s Compressor Power : = m ( h2 − h1 ) = 1 (kg ) X ( 220.0 − 183.1) kJ / kg = 36.9 kJ / min = 0.615 kW ….ANS A N Khudaiwala,G.P.Porbandar Example 9 A Freon-12 refrigerator producing a cooling effect of 20 kJ/sec operates on a simple cycle with pressure limits of 1.509 bar and 9.607 bar. The vapour leaves the evaporator dry saturated and there is no undercooling. Determine the power required by the machine. If the compressor operates at 300 rpm and has a clearance volume of 3% of strokeTemp volume,Pdetermine piston displacement of the compressor. For compressor vg the Enthalpy Enthalpy Entropy Entropy Specific s assume that (bar) the expansion the law (oC) hf hg PV1.3 = Constant. sf sg heat (m3/kg) following (kJ/kg) (kJ/kg) (kJ/kg) (kJ/kg) (kJ/kg.K) -20 1.509 0.1088 17.8 178.61 0.073 0.7082 --- } 9.607 --- 74.53 203.05 0.2716 0.682 0.747 40 Given : Tcond = 40 ºC Tevap = -20 ºC x1 = 1….dry saturated vapour h2 = 220 kJ/kg A N Khudaiwala,G.P.Porbandar hf1 = 17.8 kJ/kg hg1 = h1 = 178.61 kJ/kg hf2 = 74.53 kJ/kg hg2’ = 203.05 kJ/kg hf3 = h4 = 74.53 kJ/kg Example 9….contd • Refrigerating Capacity : = m ( h1 − h4 ) • ⇒ 20 kW = m X (178.61 − 74.53) kJ / kg • Temperature, T ⇒ m = 0.192 kg / sec 2 313 K 3 Isentropic Compression : 1-2 s1 = s2 2’ T2 s1 = s2 ' + C P ln T2' 253 K 1 4 g Sat. Liq. Line f Entropy, s T 0.7082 = 0.682 + ( 0.747 ) ln 2 313 Sat. Vapour Line ⇒ T2 = 324.2 K h2 = h2' + C P ( T2 − T2 ' ) = 203.05 (kJ / kg ) + ( 0.747 kJ / kg.K ) ( 324.2 − 313.0 ) K = 211.4 kJ / kg A N Khudaiwala,G.P.Porbandar Example 9 • Power Required : = m ( h2 − h1 ) = 0.192 ( kg / sec) X ( 211.4 − 178.61) kJ / kg Temperature, T = 6.29 kW ….ANS Vol. Efficiency : 2 313 K 3 2’ Sat. Vapour Line 1 4 Sat. Liq. Line 1 / 1.13 253 K g η vol Pd = 1 + k − k PS 1/ n f Entropy, s 9.607 bar = 1 + 0.03 − 0.03 1.509 bar = 87.6 % • Vol of Refrigerant = m ∗ v g at Intake : = 0.192 (kg / sec) X 0.1088 (m 3 / kg ) = 0.02089 m 3 / sec ( Actual Vol.) 0.02089 (m 3 / sec) ∗ 60 (sec/ min) = = Piston Displ. Vol. : η vol ∗ (rpm) A N Khudaiwala,G.P.Porbandar 0.876 ∗ 300 (rpm) = 0.00477 m 3 ….ANS Example 10 A food storage locker requires a refrigeration capacity of 50 kW. It works between a condenser temperature of 35 ºC and an evaporator temperature of -10 ºC. The refrigerator is ammonia. It is sub-cooled by 5 ºC before entering the expansion valve. By the dry saturated vapour leaving the evaporator. Assuming a single-cylinder, single-acting compressor operating at 1000 rpm with stroke equal to 1.2 times the bore, determine : 1. The power required. 2. The cylinder dimensions. Properties of ammonia are : Sat. Pr. Enthalpy Entropy Sp. Vol. Sp. Heat Temp. (oC) (bar) (kJ/kg) (kJ/kg) (m3/kg) (kJ/kg.K) Liquid Vapour Liquid Vapou r Liquid Vapour Liquid Vapour -10 2.9157 154.056 1450.22 0.82965 5.7550 --- 0.417477 --- 2.492 35 13.522 366.072 1488.57 1.56605 5.2086 1.7023 0.095629 4.556 2.903 Given : } Tcond = 35 ºC Tevap = -10 ºC x1 = 1….dry saturated vapour State 3 = Sub-cooled by 5 ºC A N Khudaiwala,G.P.Porbandar h1 = 1450.22 kJ/kg h2’ = 1488.57 kJ/kg hf3 = 366.072 kJ/kg Temperature, T Example 10….contd 2 3 308 K 303 K Isentropic Compression : 1-2 2’ 3’ 263 K T2 s1 = s2 ⇒ s1 = s2 ' + C P ln T2 ' T2 h = h = h − C ( T 5−.T755) = 5.2086 + ( 2.903) ln 1= 366.07 (kJ / kg ) − 405.56 ( 308 − 303) (kJ / kg ) 308 Sat. Vapour Line = 343.29 kJ / kg ⇒ T2 = 371.8 K 3' 4 g Sat. Liq. Line f 4 f3 P liq sat subcool Entropy, s h2 = h2' + C P ( T2 − T2' ) = 1488.57 ( kJ / kg ) + ( 2.903 kJ / kg.K ) ( 371.8 − 308.0 ) K = 1673.8 kJ / kg ME0223 SEM-IV A N Khudaiwala,G.P.Porbandar Applied Thermodynamics & Heat Engines Example 10….contd Temperature, T Mass of Refrigerant : 303 K m= 50 (kW ) 50 (kW ) = ( h1 − h4 ) kJ / kg (1450.22 − 343.29) kJ / kg = 0.04517 kg / sec 2 308 K 3 • 2’ Compressor Power : • = m ( h2 − h1 ) 3’ = 0.04517 (kg ) X (1673.8 − 1450.22 ) kJ / kg 263 K 1 4 g Sat. Liq. Line = 10.1 kW ….ANS Sat. Vapour Line f Cylinder Dimensions : 1000 (rpm) π 2 N π 2 D L D (1.2 D) Entropy, • s 4 60 4 60 m = 0.04517 (kg / sec) = = vg 0.417477 m 3 / kg ( ⇒ ⇒ ME0223 SEM-IV D = 0.19 m ….ANS L =1.2 ∗ (0.19 m) = 0.228 m ….ANS A N Khudaiwala,G.P.Porbandar Applied Thermodynamics & Heat Engines ) Vapour Absorption Refrigeration system In this system compression process of vapour compression cycle is eliminated. Instead of that the following three processes are carried out. 1. Absorbing ammonia vapour into water. 2. Pumping this solution to a high pressure cycle 3. Producing ammonia vapours from ammonia solution by heating. A N Khudaiwala,G.P.Porbandar Vapour Absorption Refrigeration system Construction: The vapour absorption system consists of a condenser, an expansion valve and an evaporator. They perform the same as they do in vapour compression method. In addition to these, this system has an absorber, a heat exchanger, an analyser and a rectifier. A N Khudaiwala,G.P.Porbandar Vapour Absorption Refrigeration system Dry ammonia vapour at low pressure passes in to the absorber from the evaporator. 2. In the absorber the dry ammonia vapour is dissolved in cold water and strong solution of ammonia is formed. 3. Heat evolved during the absorption of ammonia is removed by circulating cold water through the coils kept in the absorber. 4. The highly concentrated ammonia (known as Aqua Ammonia) is then pumped by a pump to generator through a heat exchanger. 1. A N Khudaiwala,G.P.Porbandar Vapour Absorption Refrigeration system 6. In the heat exchanger the strong ammonia solution is heated by the hot weak solution returning from the generator to the absorber. 7. In the generator the warm solution is further heated by steam coils, gas or electricity and the ammonia vapour is driven out of solution. 8. The boiling point of ammonia is less than that of water. 9. Hence the vapours leaving the generator are mainly of ammonia. A N Khudaiwala,G.P.Porbandar Vapour Absorption Refrigeration system 9. The weak ammonia solution is left in the generator is called weak aqua. 10. This weak solution is returned to the absorber through the heat exchanger. 11. Ammonia vapours leaving the generator may contain some water vapour. 12. If this water vapour is allowed to the condenser and expansion valve, it may freeze resulting in chocked flow. 13. Analyser and rectifiers are incorporated in the system before condenser. A N Khudaiwala,G.P.Porbandar Vapour Absorption Refrigeration system 14. The ammonia vapour from the generator passes through a series of trays in the analyser and ammonia is separated from water vapour. 15. The separated water vapour returned to generator. 16. Then the ammonia vapour passes through a rectifier. 17. The rectifier resembles a condenser and water vapour still present in ammonia vapour condenses and the condensate is returned to analyser. 18. The virtually pure ammonia vapour then passes through the A N Khudaiwala,G.P.Porbandar condenser. Vapour Absorption Refrigeration system 19. The latent heat of ammonia vapour is rejected to the cooling water circulated through the condenser and the ammonia vapour is condensed to liquid ammonia. 20. The high pressure liquid ammonia is throttled by an expansion valve or throttle valve. 21. This reduces the high temperature of the liquid ammonia to a low value and liquid ammonia partly evaporates. 22. Then this is led to the evaporator. 23. In the evaporator the liquid A N Khudaiwala,G.P.Porbandar Vapour Absorption Refrigeration system 24. The latent heat of evaporation is obtained from the brine or other body which is being cooled. 25. The low pressure ammonia vapour leaving the evaporator again enters the absorber and the cycle is completed. 26. This cycle is repeated again to provide the refrigerating effect. A N Khudaiwala,G.P.Porbandar Comparison between Vapour compression & Vapour Absorption refrigeration systems S.No. Vapour Compression System Vapour Absorption System 1 This system has more wear and Only moving part in this system is tear and produces more noise due an aqua pump. Hence the quieter to the moving parts of the in operation and less wear and tear compressor. 2. Electric power is needed to drive the system Waste of exhaust steam may be used. No need of electric power 3. Capacity of the system drops rapidly with lowered evaporator pressure Capacity of the system decreases with the lowered evaporative pressure, by increasing the steam pressure in generator. 4. At partial loads performance is poor. At partial loads performance is not affected. 5. Mechanical energy is supplied through compressor Heat energy is utilised 6. Energy supplied is ¼ to ½ of the Energy supplied is about one and A N Khudaiwala,G.P.Porbandar refrigerating effect half times the refrigerating effect Comparison between Vapour compression & Vapour Absorption refrigeration systems S.No. Vapour Compression System Vapour Absorption System 7. Charging of the refrigerating to the system is easy Charging of refrigerant is difficult 8. Preventive measure is needed, since liquid refrigerant accumulated in the cylinder may damage to the cylinder Liquid refrigerant has no bad effect on the system. A N Khudaiwala,G.P.Porbandar Layout of Domestic Refrigerator A N Khudaiwala,G.P.Porbandar Window Type Air Conditioner A N Khudaiwala,G.P.Porbandar Window Type Air Conditioner This is also called room air conditioner. This unit consists of the following. 1. A cooling system to cool and dehumidify the air involves a condenser, a compressor and a refrigerant coil. 2. A filter to any impurities in the air. The filter is made of mesh, glass wool or fiber. 3. A fan and adjustable grills to circulate the air. 4. Controls to regulate the equipment operation. A N Khudaiwala,G.P.Porbandar Window Type Air Conditioner The low pressure refrigerant vapour is drawn from the evaporator to the hermetic compressor through suction pipe. It is compressed from low pressure to the high pressure and supplied to the condenser. It is condensed in the condenser by passing the outdoor air over the condenser coil by a fan. The liquid refrigerant is passed through the capillary into the evaporator. A N Khudaiwala,G.P.Porbandar Window Type Air Conditioner 5. In the evaporator the liquid refrigerant picks up the heat from the refrigerator surface and gets vaporized. 6. A motor driven fan draws air from the room through the air filter and this air is cooled by losing its heat to the low temperature refrigerant and cold air is circulated back into the room. 7. The vapour refrigerant from the evaporator goes to the compressor from evaporator and the cycle is repeated. 8. Thus the room is air conditioned A N Khudaiwala,G.P.Porbandar Window Type Air Conditioner 9. The quantity of air circulated can be controlled by the dampers. 10. The moisture in the air passing over the evaporator coil is dehumidified and drips into the trays. 11. This water evaporator to certain extent and thus helps in cooling the compressor and condenser. 12. The unit automatically stops when the required temperature is reached in the room. This is accomplished by the A N Khudaiwala,G.P.Porbandar thermostat and control panel. Split Type Air Conditioner - Construction A N Khudaiwala,G.P.Porbandar Thermodynamic and thermo-physical properties of Refrigerants a) Suction pressure: Higher suction pressure is better as it leads to smaller compressor displacement. b) Discharge pressure: At a given condenser temperature, the discharge pressure should be as small as possible to allow light-weight construction of compressor, condenser etc. c) Pressure ratio: Should be as small as possible for high volumetric efficiency and low power consumption. d) Latent heat of vaporization: Should be as large as possible so that the required mass flow rate per unit cooling capacity will be small. A N Khudaiwala,G.P.Porbandar Thermodynamic and thermo-physical properties of Refrigerants e) Isentropic index of compression: Should be as small as possible so that the temperature rise during compression will be small. f) Liquid specific heat: Should be small so that degree of subcooling will be large leading to smaller amount of flash gas at evaporator inlet. g) Vapour specific heat: Should be large so that the degree of superheating will be small. h) Thermal conductivity: Thermal conductivity in both liquid as well as vapour phase should be high for higher heat transfer coefficients. i) Viscosity: Viscosity should be small in both liquid and vapour phases for smaller frictional pressure drops A N Khudaiwala,G.P.Porbandar Environmental and safety properties a) Ozone Depletion Potential (ODP): According to the Montreal protocol, the ODP of refrigerants should be zero, i.e., they should be non-ozone depleting substances. Refrigerants having non-zero ODP have either already been phased-out (e.g. R 11, R 12) or will be phased-out in near-future(e.g. R22). Since ODP depends mainly on the presence of chlorine or bromine in the molecules, refrigerants having either chlorine (i.e., CFCs and HCFCs) or bromine cannot be used under the new regulations b) Global Warming Potential (GWP): Refrigerants should have as low a GWP value as possible to minimize the problem of global warming. Refrigerants with zero ODP but a high value of GWP (e.g. R134a) are likely to be regulated in future. A N Khudaiwala,G.P.Porbandar Environmental and safety properties c) Total Equivalent Warming Index (TEWI): The factor TEWI considers both direct (due to release into atmosphere) and indirect (through energy consumption) contributions of refrigerants to global warming. Naturally, refrigerants with as a low a value of TEWI are preferable from global warming point of view. d) Toxicity: Ideally, refrigerants used in a refrigeration system should be non-toxic.Some fluids are toxic even in small concentrations. Some fluids are mildly toxic, i.e., they are dangerous only when the concentration is large and duration of exposure is long. A N Khudaiwala,G.P.Porbandar Environmental and safety properties e) Flammability: The refrigerants should preferably be non-flammable and non-explosive. For flammable refrigerants special precautions should be taken to avoid accidents. f) Chemical stability: The refrigerants should be chemically stable as long as they are inside the refrigeration system. g) Compatibility :with common materials of construction (both metals and non-metals) h) Miscibility with lubricating oils: Oil separators have to be used if the refrigerant is not miscible with lubricating oil (e.g. ammonia). Refrigerants that are completely miscible with oils are easier to handle(R12). A N Khudaiwala,G.P.Porbandar Halocarbon Refrigerants Halocarbon Refrigerants are all synthetically produced and were developed as the Freon family of refrigerants. Examples : CFC’s : R11, R12, R113, R114, R115 HCFC’s : R22, R123 HFC’s : R134a, R404a, R407C, R410a A N Khudaiwala,G.P.Porbandar Inorganic Refrigerants Carbon Dioxide Water Ammonia Air Sulphur dioxide A N Khudaiwala,G.P.Porbandar R22 ODP-0.05, GWP-1700 R22 has 40% more refrigerating capacity Higher pressure and discharge temp and not suitable for low temp application Extensively used in commercial air- conditioning and frozen food storage and display cases A N Khudaiwala,G.P.Porbandar R123 ODP-0.02,GWP-90 As a replacement for R11 as similar thermodynamic properties. Very short atmospheric life but classified as carcinogen Retrofit alternative to R11 A N Khudaiwala,G.P.Porbandar HFC Zero ODP as no chlorine atom contains only Hydrogen and Flurodine Very small GWP values No phase out date in Montreal Protocol R134a and R152 a – Very popular refrigerants HFC refrigerants are costly refrigerants A N Khudaiwala,G.P.Porbandar R134a ODP-0, GWP-1300 Used as a substitute for R12 and to a limited range for R22 Good performance in medium and high temp application Toxicity is very low Not miscible with mineral oil A N Khudaiwala,G.P.Porbandar Ammonia ODP = 0 and GWP = 0 Excellent thermodynamic characteristics: small molecular mass, large latent heat, large vapour density and excellent heat transfer characteristics High critical temperature (132C) : highly efficient cycles at high condensing temperatures Its smell causes leaks to be detected and fixed before reaching dangerous concentration Relatively Low price A N Khudaiwala,G.P.Porbandar Drawbacks of Ammonia Toxic Flammable ( 16 – 28% concentration ) Not compatible with copper Temperature on discharge side of compressor is higher compared to other refrigerants A N Khudaiwala,G.P.Porbandar Water Zero ODP & GWP Water as refrigerant is used in absorption system .New developing technology has created space for it for use in compression cycles also. But higher than normal working pressure in the system can be a factor in restricted use of water as refrigerant. A N Khudaiwala,G.P.Porbandar Application of New Eco-friendly Refrigerants Application of Refrigerants HFCs used Eco-friendly refrigerant Domestic refrigeration R134a,R152a HC600a and blends Commercial refrigeration ,CO2 R134a,R404A,R407C HC blends,NH3 ,CO2 ** Cold storage ,food processing And industrial refrigeration R134a,R404A,R507A NH3 ,HCs,CO2 ** Unitary air conditioners R410A,R407C CO2 , HC s Centralized AC (chillers) R134a,R410A,R407C NH3 ,HCs,CO2, water ** Transport refrigeration R134a,R404A CO 2, Mobile air conditioner R134a CO2 ,HCs A N Khudaiwala,G.P.Porbandar A N Khudaiwala,G.P.Porbandar