Refrigeration Cycle

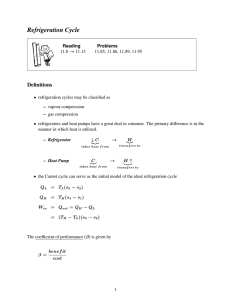
Refrigeration Cycle
Reading
11-1 → 11-7, 11-9
Problems
11-11, 11-46, 11-49, 11-103
Definitions
• the 1st law of thermodynamics tells us that heat flow occurs from a hot source to a cooler sink, therefore, energy in the form of work must be added to the process to get heat to flow from a low temperature region to a hot temperature region.
• refrigeration cycles may be classified as
– vapour compression
– gas compression
• refrigerators and heat pumps have a great deal in common. The primary difference is in the manner in which heat is utilized.
– Refrigerator ↓ C
| {z } takes heat f rom
→ H
|{z} transf ers to
– Heat Pump C
|{z} takes heat f rom
→ H ↑
| {z } transf ers to
1
The coefficient of performance (COP) is given by
COP = benef it cost where the benefit for a refrigeration process is the cooling load given as Q
L
. This is the net benefit, i.e. heat is removed from the cold space. For a heat pump, the benefit is the heat added to the hot space, i.e.
Q
H
.
COP ref rig
=
Q
L
W in
=
Q
L
Q
H
− Q
L
=
Q
H
1
− 1
Q
L
=
1
T
H
( s
4
T
L
( s
3
− s
1
)
− 1
− s
2
)
=
T
H
T
L
1
− 1
T
L
=
T
H
− T
L
COP heat pump
=
Q
H
W in
=
Q
H
Q
H
− Q
L
1
=
Q
L
1 −
Q
H
1
=
T
L
1 −
T
H
=
T
H
T
H
− T
L
2
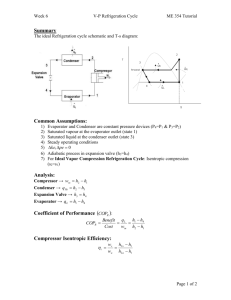
Vapour Compression Refrigeration Cycle
Expansion
Valve sat. liquid
Room Air
Q
H
Condenser superheated vapour compressor
gas
3
2 phase
Evaporator
Food
Q
L
sat. vapour
3
Refrigeration Process
Process
1-2s:
2s-3:
3-4
4-1
Description
A reversible, adiabatic (isentropic) compression of the refrigerant.
The saturated vapour at state 1 is superheated to state 2.
⇒ w c
= h
2 s
− h
1
An internally, reversible, constant pressure heat rejection in which the working substance is desuperheated and then condensed to a saturated liquid at 3. During his process, the working substance rejects most of its energy to the condenser cooling water.
⇒ q
H
= h
2 s
− h
3
An irreversible throttling process in which the temperature and pressure decrease at constant enthalpy.
⇒ h
3
= h
4
An internally, reversible, constant pressure heat interaction in which the working fluid is evaporated to a saturated vapour at state point 1. The latent enthalpy necessary for evaporation is supplied by the refrigerated space surrounding the evaporator.
The amount of heat transferred to the working fluid in the evaporator is called the refrigeration load.
⇒ q
L
= h
1
− h
4
4
Common Refrigerants
There are several fluorocarbon refrigerants that have been developed for use in VCRC.
R11
R12
R22
R134a
R141b
Ammonia
R744
R290
CCl
2
F
2 dichlorofluoromethane
- used for refrigeration systems at higher temperature levels
- typically, water chillers and air conditioning
CHClF
2 has less chlorine, a little better for the environment than R12
- used for lower temperature applications
CF H
2
CF 3 tetrafluorethane - no chlorine
- went into production in 1991
- replacement for R12
C
2
H
3
F Cl
2 dichlorofluoroethane
N H
3 corrosive and toxic
- used in absorption systems
CO
2 propane behaves in the supercritical region
- low efficiency combustible
5
Designation Chemical Ozone Depletion Global Warming
Formula Potential 1 Potential 2
Ozone Depleting & Global Warming Chemicals
CFC-11
CFC-12
CFC-13
CFC-113
CFC-114
CFC-115
Halon-1211
Halon-1301
Halon-2402 carbon tetrachloride methyl chloroform nitrous oxide
CCl
3
F
CCl
2
F
2
CClF
3
C
2
F
3
Cl
3
C
2
F
4
Cl
2
C
2
F
5
Cl
1
CF
2
ClBr
CF
3
Br
C
2
F
4
Br
2
CCl
4
CH
3
CCl
3
N
2
O
1
0.89
0.81
0.69
0.32
2.2-3.5
8-16
5-6.2
1.13
0.14
3,400
7,100
13,000
4,500
7,000
7,000
4,900
1,300
270
Ozone Depleting & Global Warming Chemicals - Class 2
HCFC-22
HCFC-123
HCFC-124
HCFC-125
HCFC-141b
HCFC-142b
CHF
2
Cl
C
2
HF
3
Cl
2
C
2
HF
4
Cl
C
2
HF
5
C
2
H
3
F Cl
2
C
2
H
3
F
2
Cl
0.048
0.017
0.019
0.000
0.090
0.054
Global Warming, non-Ozone Depleting Chemicals carbon dioxide methane
HFC-125
HFC-134a
HFC-152a
CO
2
CH
4
CHF
2
CF
3
CF H
2
CF
3
CH
3
CHF
2 perfluorobutane perfluoropentane perfluorohexane
C
4
F
10
C
5
F
12
C
6
F
14 perfluorotributylamine N ( C
4
F
9
)
3
0
0
0
0
0
0
0
0
0
1,600
90
440
3,400
580
1800
1
11
90
1,000
2,400
5,500
5,500
5,100
4,300
1 - relative to R11
2 - relative to CO
2
6
Cascade Refrigeration System
• two or more vapour compression refrigeration cycles are combined
• used where a very wide range of temperature between T
L and T
H is required
Advantages
• the refrigerants can be selected to have reasonable evaporator and condenser pressures in the two or more temperature ranges
Q
L
( ↑ )
COP =
W net
( ↓ ) overall ( ↑ )
7
Absorption Refrigeration System
Differences between an absorption refrigeration system and a VCRC
VCRC
• vapour is compressed between the evaporator and the condenser
• process is driven by work
•
Absorption RS the refrigerant is absorbed by an absorbent material to form a liquid solution
• heat is added to the process to retrieve the refrigerant vapour from the liquid solution
• process is driven by heat
8
Process
liquid ammonia
Room Air
Q
H
Condenser
Expansion
Valve ammonia vapour only
Source of
Heat
Q
*
H
Generator weak ammonia water solution cold regenerator
2 phase
Evaporator
Food
Q
L dry vapour
Absorber cold sink
Q
*
L strong ammonia water solution pump
9