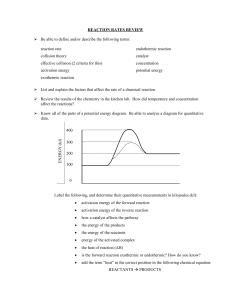
Controlling Chemical Reactions Science 8 https://youtu.be/YCsOAnv-lSg You are working on an engineering team that tears down buildings. “3, 2, 1……Let it go!” You push a button and suddenly a loud rumbling sound starts. The ground shakes, and clouds of dust pour into the street. In 15 seconds, a tall building is reduced to a pile of rocks. Careful control of energy in the explosion is critical to collapse a building without even breaking a window next door. If you don’t understand the chemical reactions used, people could get hurt or property could get damaged. You know, you may never blow up buildings, but you use energy from controlled chemical reactions every day. Every time your body changes your lunch to energy to play sports, you are using controlled reactions. Getting Reactions Started Have you ever been out in the woods and tried to start a fire? Sometimes they are almost impossible to start. You’re camping, you’re cold, it’s raining. You want that fire and you want it NOW! Why is it so hard to start some chemical reactions? Activation Energy Think of a rock at the top of a hill. That rock contains stored energy because of its position. It just sits there and remains motionless until it’s pushed over a small hump. Well, after that, it just falls down the hill rapidly, and releases its stored energy. https://youtu.be/Zlu7FhfMBWk Every chemical reaction is like that rock. The reaction won’t begin until the reactants get the energy needed to push them “over the hump.” Chemical reactions need a certain amount of energy to get started. Energy is needed to break existing chemical bonds. Once the energy is available, the atoms begin to form the new chemical bonds that create the products. The minimum amount of energy needed to a start chemical reaction is called ACTIVATION ENERGY of the reaction. Think about when hydrogen and oxygen form water. This chemical change gives off tremendous amounts of energy. You can mix the two gases together and the mixture can remain unchanged for years. For a reaction to start, a tiny amount of activation energy-just a spark- is needed. Once a few molecules of hydrogen and oxygen react, the rest will follow because each reaction provides activation energy for new reactions. Do reactions need energy to keep going? That answer depends on whether the reaction is exothermic or endothermic. Energy and Types of Reactions See graphs You've learned that an exothermic reaction is one that gives off energy. Most chemical reactions are exothermic. They follow the pattern seen in the graph above. Notice that the dotted line shows the energy levels of the reactants before the reaction. Like all reactions, exothermic reactions need activation energy to get started. But additional energy is not needed to complete the reaction. As a result, energy is given off as the reaction takes place. This results in the energy level of the products being lower than the energy level of the starting materials. An example of an exothermic reaction is burning fuel to produce heat. In the other graph you see an example of the energy produced in an endothermic reaction. It also needs activation energy to get started. However, it needs a supply of energy to keep going. Because the materials absorb energy as the products are formed, the energy level of the final materials is higher than that of the starting materials. That’s what happens when baking soda and vinegar react. Rates of Chemical Reactions https://youtu.be/NhdtqnEfa9w As you may have seen, chemical reactions don’t all happen at the same rate. Some, like explosions, are very fast. Others, like rusting of metal, are much slower. Some reactions can happen at different rates depending what the conditions are. If you want to make a chemical reaction happen faster, you need to get more reactant particles together more often. To slow down a reaction, you need to do the opposite-get fewer particles together less often. Chemists can control the rates of reactions by changing factors such as concentration, temperature, and surface area, and by using substances called catalysts and inhibitors. Concentration One way to increase the rate of reaction is to increase the concentration of the reactants. Concentration is the amount of one material in a given amount of another material. For example, adding a spoonful of sugar to a glass of lemonade will make it sweet. BUUUUUUt, adding a large spoon of sugar makes the lemonade a lot sweeter! The glass with greater amount of sugar has a greater concentration of sugar molecules. Increasing the concentration of the reactants makes more particles available to react. Temperature Another way to increase the reaction is to increase the temperature. When you heat a substance, its particles move faster. Faster- moving particles increase the reaction rate in two ways. ● First, the particles come in contact more often, which means there are more chances for a reaction to happen. ● Second, faster moving particles have more energy. The energy helps the reactants get over the activation energy “hump.” Suppose you forgot to put the milk back in the refrigerator before you went to school. When you get home the milk smells sour. Milk contains bacteria, which carries out thousands of chemical reactions as they live and reproduce. At room temperature, those reactions happen quickly. Some of those reactions cause food to spoil. Keeping food cold slows down those reactions, so your food stays fresh longer. Surface Area When a chunk of a solid reacts with a liquid or a gas, only the particles on the surface of that solid can come into contact with the other reactant. Now, what if you break that chunk into smaller pieces? What happens? You have increased your surface area! More particles of the material are exposed, so the reaction happens faster. Catalysts Another way to control the rate of a reaction is to change the activation energy. If you decrease the activation energy, the reaction happens faster. https://youtu.be/m_9bpZep1QM A catalyst is a material that increases the rate of a reaction by lowering the activation energy. Catalysts help change a reaction’s rate, they are not permanently changed in the reaction. Catalysts are themselves not considered reactants. The cells in your body contain biological catalysts called enzymes. Enzymes provide a surface on which reactions take place. The surface helps reactions happen at lower temperatures because it lowers activation energy. After the reaction, the enzyme breaks away unchanged. Inhibitors Sometimes a reaction is more helpful if it can be speeded up instead of slowed down. There is a material used to decrease the rate of reaction called an inhibitor. Inhibitors work in many ways. https://study.com/academy/lesson/inhibitors-definition-types-qui z.html Section Review Answer the questions 1. What would happen if the activation energy for a particular chemical was not available? 2. Describe three ways to increase the rate of a chemical reaction. 3. Which has greater surface area: a sugar cube of an equal mass of sugar crystals? Explain.