Changes of State

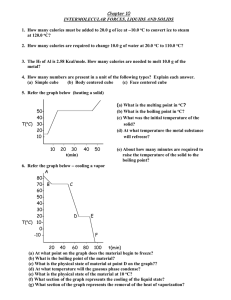
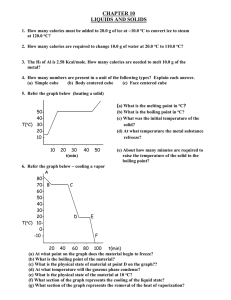
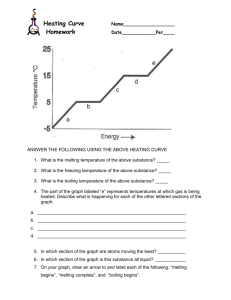
Heating and Cooling Curves
Phase Diagrams
Objectives
Discuss the following:
evaporation condensation molar heat of vaporization molar heat of fusion boiling point melting point supercooling sublimation deposition molar heat of sublimation phase changes critical temperature and pressure
Sketch a typical heating curve and identify various aspects of it.
Use phase diagrams to identify what phase(s) is/are present given specific conditions.
Phases
A phase is a homogeneous part of a system in contact with other parts of the system but with a distinct boundary
Vapor is the gaseous state of a substance that is normally a solid or liquid at that temperature or pressure
In a closed system a dynamic equilibrium is reached between the vapor and the condensed phase
As many molecules enter the gas phase as return to the condensed phase
Changes of State
Deposition
Solid
Melting
Liquid
Evaporation
Boiling
Gas
Freezing Condensation
Sublimation
Heating Curve
Melting
Boiling
Nomenclature
The melting point of a solid or the freezing point of a liquid is the temperature at which the solid and liquid phases coexist in equilibrium
These are pressure dependent!
The boiling point is the temperature at which the
(equilibrium) vapor pressure of a liquid is equal to the external pressure.
The normal boiling point is the temperature at which a liquid boils when the external pressure is 1 atm.
Heating increases kinetic energy, potential energy and vibrational and rotational energy
Heating increases potential energy and for vaporization does PV work
Phase Diagram of Water
A phase diagram summarizes the conditions at which a substance exists as a solid, liquid, or gas.
Phase Diagrams-Water
Water is unique!
Density of solid is less than density of liquid so ice floats
As pressure increases, melting point decreases
Triple point:
Only combination of temperature and pressure where all three phases exist at one time
At the critical point separate liquid and gas phases cease to exist
Triple Point
Dry ice is dry because it sublimes at 1 atm pressure