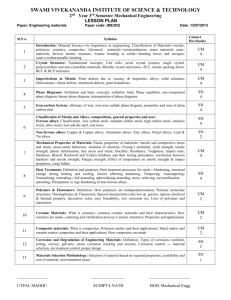
Ferrous and non-ferrous materials Dr Bertram Mallia Room 221, Faculty of Engineering bertram.mallia@um.edu.mt 1 Course description: Introduction to a wide range of ferrous and non-ferrous materials The role of alloying elements and other factors effecting the properties of these materials 2 Suggested reading • Engineering Materials by R. L. Timing, Volume 1, second edition, ISBN 978-0-582-31928-8 • Light alloys by Ian Polmear, fourth edition, ISBN 0-7506-6371-5 • Physical Metallurgy by William F. Hosford, ISBN 0-8247-2321-6 • Steels Microstructure and Properties by R.W.K Honeycombe and H.K.D.H. Bhadeshia, second edition, ISBN 0-340-58946-9 • Materials Science and Engineering by W.D. Callister, sixth edition, ISBN 0-471-22471-5 3 Course material: Website: http://www.um.edu.mt/vle 4 Engineering Materials Ferrous metals Metals Engineering materials Non-metallic materials Nonferrous metals Synthetic materials Natural materials 5 Ferrous Alloys • Alloys based on metallic element iron (Latin – ferrum) • Iron is rarely found in pure state ; It converts to oxide in a matter of few years • Engineers usually find it associated with carbon (non-metal) • Although all Fe-C alloys can arguably be considered as ferrous alloys, these are referred to as Wrought iron, plain carbon steel and plain cast irons (depending on C content and the way it is associated with iron content) • The term ferrous alloys is reserved for ferrous materials containing additional metallic alloying elements in sufficient quantities to modify the properties of the material 6 Ferrous metals Carbon steel (low) Classification: Carbon steel (meduim) Carbon steel (high) Steel Alloy steel Grey cast iron Ferrous material Cast iron White cast iron Malleable cast iron Spheriodal cast iron Wrought iron Alloy cast iron 7 Non-Ferrous metals Aluminium Cadmium Chromium Cobalt Copper Gold Lead Magnesium Manganese Molybdenium Nickel Platinium Silver Tin Titanium Vanadium Zinc Metals NonFerrous material Brass (copper and zinc) Phosphor bronze Tin bronze Gun metal Aluminium bronze (Cu-Al) Cupro-nickel alloys (Cu-Ni) HT (wrought) Aluminium alloys HT (cast) Non HT (wrought) Non HT (cast) Magnesium alloys Alloys Zinc based ‘die casting’ alloys Soft solders Tin-lead solders 8 Bearing metals ...... all metals not classified as ferrous • Pure non-ferrous metals Generally have poor mechanical properties and are used for applications where special properties are required Ex corrosion resistance (Cu, Al, Pb, Zn); electric conductivity (Cu, Al), thermal conductivity (Cu, Al) 9 Non- ferrous metals Metal Density (kg/m3) Tm (oC) Properties Uses Aluminium 2700 660 Lightest of commonly used metals, high electrical and thermal conductivity, Soft, ductile and low TS (93MPa) Base of many engineering alloys, lightweight electrical conductors Copper 8900 1083 Soft, ductile, low TS (232MPa), High conductivity (2nd to silver), easier to join by brazing or soldering than Al. Base of brass and bronze alloys, electrical conductors and heat exchangers Lead 11 300 328 Soft ductile, very low TS, high corrosion resistance Electric cable sheets, base of ‘solder alloys’, lining in chemical plants, added to other metals to make them free cutting 10 Metal Density (kg/m3) Tm (oC) Properties Uses Titanium 4510 1678 Good creep, excellent corrosionresistant Base of many engineering alloys, Aerospace, chemical industry, biomedical, power generation, automotive, marine, sports Silver 10 500 960 Soft, ductile, and very Used in electrical and low TS. Highest electronic engineering for electrical conductivity switch and relay contacts of metals Tin 7 300 232 Corrosion resistant Tin plate, soft solders, one of the bases for ‘white metal’ bearings, An alloying element in bronzes. 11 Metal Density (kg/m3) Tm (oC) Properties Uses Zinc 7100 420 Soft, ductile and low TS, Corrosion resistant Alloying element in brass, galvanising, base of die casting alloys Chromium 7500 1890 Resists corrosion, Increase strength but lowers ductility of steels. Improves heat treatment Alloying element in high strength and corrosion resistant steels, electroplating Cobalt 8900 1495 Improves wear resistance and hot hardness of high speed steels Alloying element in high speed steels and in permanent magnet alloys 12 Metal Densit y (kg/m3) Tm (oC) Properties Uses Manganese 7200 1260 High affinity for oxygen and sulphur De-oxidise steel, offset ill effects of sulphur impurities, large amounts improve wear resistance Molybdenum 9550 2620 A heavy heat resistant metal that alloys readily with other metals Alloying element in high strength nickel-chrome steels to improve mechanical and heat treatment prop. Reduces mass effect and temper brittleness Nickel 8900 1458 Strong, tough, corrosion resistant metal Alloying element to improve strength, and mechanical properties of steel. Tends to unstabilise carbon during heat treatment and Cr has to be added to counter this effect in medium and high carbon steels 13 • Non-ferrous alloys Copper alloys: Bronze (Cu-Sn) – High corrosion resistance; easily machined; relative high melting temperature. Heavy and expensive compared to ferrous materials Applications: steam and hydraulic valve components; marine applications 14 • Brass (Cu and Zn) – Weaker and less corrosion resistant compared to bronzes. Easily hot formed and can be easily machined to good finish. Applications: Manufacture of electrical components and domestic water fittings. 15 • Aluminium alloys Generally less strong than ferrous and copperbased alloys however they are lighter in weight. More corrosion resistant than ferrous materials (except stainless steels). The strength of these alloys fall rapidly with temperature 16 • Titanium alloys Strong as steel and light in weight as aluminium. Titanium retains its strength at high temperatures and is very corrosion resistant. On the negative side, titanium is very costly and is difficult to shape 17 Group Project • 8 groups • Deliverables : (1) Present a group report on an assigned ferrous or non-ferrous material (2) Presentation (23 mins) (Carry 15% of the exam mark) 18 Group 1 • Tool steels - Introduction Main groups of tool steels Alloy design and heat treatment Shock resistant tool steels; Hot work tool steels; High speed steels; Cold work tool steels; Mold steels .... - Applications 19 Group 2 • - Cast iron Into/ History Iron-carbon system Types of cast iron (Grey cast Iron, White cast Iron, Spheroidal cast iron, Malleable cast iron, Alloy cast iron) - Effect of alloying elements/ impurities and cooling rate on microstructure development - Properties - Applications 20 Group 3 • Aluminium and its alloys - History / Intro - Wrought Aluminium alloys (Heat treatable and non-heat treatable) - Cast Aluminium alloys (Heat treatable and nonheat treatable) - Hydrogen porosity - Hardening mechanisms - Properties and applications 21 Group 4 • Nickel and nickel base alloys - Introduction / Historical development - Physical metallurgy (solid solution hardening, carbide strengthening, precipitation hardening) - Various Nickel alloys such as superalloys and their properties and applications (ex. Heat resistant applications; corrosion resistance; low expansion alloys; electrical resistance alloys; soft magnetic alloys; shape memory alloys) 22 Group 5 • - Copper and copper base alloys History / Intro Commercial pure copper and characteristics High copper content alloys Cu-Zn alloys (Brasses) Cu-Sn alloys (Bronzes) Cu-Ni (cupronickel) alloys Aluminium-bronze alloys Properties and Applications 23 Group 6 • Titanium and its alloys - History / Introduction α alloys Near α alloys α + β alloys β alloys Properties and applications 24 Group 7 • Magnesium alloys - Introduction/ History Casting alloys Wrought alloys Corrosion and mechanical properties Applications 25 Group 8 • Stainless steels - Introduction / History - Effect of composition on stainless steel microstructure (ferrite/ austenite stabilsers, martensite start temperature) - Ferritic stainless steels - Martensitic stainless steels - Austenitic stainless steels - Precipitation hardening steels - Duplex stainless steels - Applications, properties and corrosion characteristics 26 Presentation date: Friday 30th October 2015 Group 1 Abdilla Amy Abela Dylan Abela Kyle Abela Warren Agius Pascalidis Gabriel Aquilina Jean Paul Azzopardi Mark Anthony Baldacchino Darrell Baldacchino Sarah Barbara Joshua Group 2 Bartolo Jamie Luke Bencini Rafel Bezzina Michel Bezzina Ryan Bonello Ylenia Victoria Borg Andrea Borg Gabriel Borg Jamie Brincat Matthew Bugeja Malcolm Group 3 Group 4 Cachia Kyrie Marie Calleja Andreas Cauchi Emanuel Cauchi George Chetcuti Cristian Coppini Michael Henry Cutajar Charise Degabriele Joseph Deguara Luke Demajo James El Sadi Yasmine Farrugia Giuseppe Farrugia Samuel Farrugia Thomas Fenech Anthea Fenech Christian Fenech Daniel Fenech Graziella Janice Galea Carl Matthew Galea Kenneth 27 Presentation date: Friday 6th November 2015 Group 5 Group 6 Gatt Nathan John Gauci Darrell Gauci Gilbert Gerada Josef Neil Grech Karl Magro Neil Mangion Ian Meilak Aaron Mercieca Dylan Mercieca Luke Mercieca Mark Mifsud Bernard Migneco Andrea-Ivan Muscat Damian Portelli Andrea Psaila Samuel Rapa Amy Saliba Christopher Saliba Christian Group 7 Group 8 Saliba Eleanor Sammut Liam Schembri Edward Jude Scicluna Marie Claire Spiteri Andrew Spiteri Luke Sultana Neil Tabone Miguel Tanti Karl Tonna Vassallo Vassallo Vella Xiberras Xuereb Xuereb Zarb Zerafa Christabelle Chantel Nicole Chelsey Adrian Aaron Damian Nicholas Jeremy 28 More Project details • 1 report (not longer than 18 A4 pages, font 12 excluding title page and references) • Indicate individual contributions • Reference work and abide with the University of Malta Plagiarism policy • Submit 1 hard and 1 soft copy • Submission deadline: Friday 27th November 2015 29