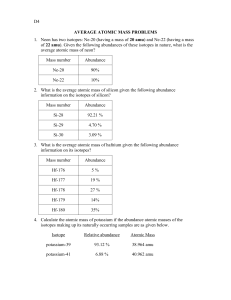
Chemistry 2.05 – 2.08 SG S1 1. Isotopes: 2. isotopes of an element have different numbers of _________________, they also have different ________________ numbers. 3. Most __________________ occur naturally as mixtures of isotopes. 4. The mass numbers on the periodic table are the ____________________ average of the most abundant isotopes’ mass numbers. 5. Atomic Mass a. b. c. d. 6. To calculate the _________________ __________________ of an element, ______________ the mass of each isotope by its natural abundance, expressed as a decimal, and then add the products. 7. Calculating Atomic Mass a. b. c. d. 8. Given the average atomic mass of an element on the periodic table and the percent natural abundance of each isotope, calculate the identity of the unknown isotope?(Atomic mass of chromium is 51.996 amu) Isotope % abundance Fraction of abundance Mass 9. Chlorine has two naturally occurring isotopes. The mass of chlorine-35 is 34.696 amu and the mass of chlorine-37 is 36.966 amu. Using the average mass from the periodic table (average atomic mass of chlorine is 35.453), find the abundance of each isotope. (Remember that the sum of the two frctional abundances must be 1) Isotope % of abundance Fraction of abundance Mass 10. The mass of a Cu-63 atom is 62.94 amu, and that of a Cu-65 atom is 64.93 amu. The percent abundance of Cu-63 is 69.17% and the percent abundance of Cu-65 is 30.83%. What is the average atomic mass? Isotope % abundance Fraction of abundance Mass 11. Calculate the average atomic mass of chromium. It is made up of isotopes with the following percent compositions and atomic masses: 83.79% with a mass of 51.94 amu; 9.50% with a mass of 52.94 amu; 4.35% with a mass of 49.95 amu; 2.36 % with a mass of 53.94 amu. Isotope % abundance Fraction of abundance Mass 12. Calculate the average atomic mass of iron if its abundance in nature is 15% iron-55 and 85% iron-56. Isotope % abundance Fraction of abundance Mass