Chemical Kinetics: Collision Theory & Reaction Rates
advertisement

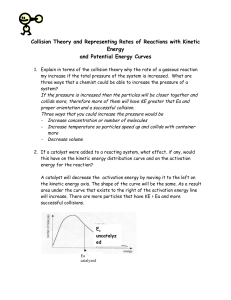
TOPIC 6 CHEMICAL KINETICS 6.1 Collision Theory and Rates of Reaction ESSENTIAL IDEA The greater the probability that molecules will collide with sufficient energy and proper orientation, the higher the rate of reaction. NATURE OF SCIENCE (2.7) The principle of Occam’s razor is used as a guide to developing a theory – although we cannot directly see reactions taking place at the molecular level, we can theorize based on current atomic models. Collision theory is a good example of this principle. INTERNATIONAL-MINDEDNESS Depletion of stratospheric ozone has been caused largely by the catalytic action of CFCs and is a particular concern in the polar regions. These chemicals are released from a variety of regions and sources, so international action and cooperation have been needed to ameliorate the ozone depletion problem. THEORY OF KNOWLEDGE The Kelvin scale of temperature gives a natural measure of the kinetic energy of gas whereas the artificial Celsius scale is based on the properties of water. Are physical properties such as temperature invented or discovered? UNDERSTANDING/KEY IDEA 6.1.A Species react as a result of collisions of sufficient energy and proper orientation. COLLISION THEORY In order to react, particles must collide with kinetic energy greater than the activation energy and have the correct collision geometry. Restating: To have a successful reaction you need 2 factors: Sufficient energy (greater than Ea) Correct geometry Endothermic Reactions PE POTENTIAL ENERGY DIAGRAM Exothermic Reactions PE POTENTIAL ENERGY DIAGRAM UNDERSTANDING/KEY IDEA 6.1.B The rate of reaction is expressed as the change in concentration of a particular reactant/product per unit time. Define the term rate of reaction. The rate of a chemical reaction is the increase in concentration of products or the decrease in concentration of reactants per unit time. Units for rate = mol dm-3s-1 2NO2(g) 2NO(g) + O2(g) Reaction Rates: 1. Can measure disappearance of reactants 2. Can measure appearance of products 3. Are proportional stoichiometrically 2NO2(g) 2NO(g) + O2(g) Reaction Rates: 4. Are equal to the slope tangent to that point [NO2] t 5. Change as the reaction proceeds, if the rate is dependent upon concentration [ NO2 ] constant t APPLICATION/SKILLS Be able to analyze graphs and numerical data from rate experiments. GUIDANCE Be able to calculate reaction rates from tangents of graphs of concentration, volume or mass vs time. GUIDANCE Be able to interpret graphs of changes in concentration, volume or mass against time. ANALYSE DATA You may be given a set of data such as concentration or changes in volume or mass vs time. You should be able to graph the data and find the rate at any point using the tangent. Be careful with units. To find the tangent at a certain point, take the change in concentration (change in “y”) divided by the change in time (change in “x”). Rates of reaction are always expressed as positive numbers even if the slope is negative. APPLICATION/SKILLS Investigate rates of reactions experimentally and evaluate the results. APPLICATION/SKILLS Be able to explain the effects of temperature, pressure/concentration and particle size on rate of reaction. Predict and explain, using the collision theory, the qualitative effects of particle size, temperature, concentration and pressure on the rate of a reaction. FACTORS AFFECTING THE RATE OF REACTION There are 5 factors which will increase the number of effective collisions. TEMPERATURE Increasing the temperature increases the kinetic energy of the particles. By increasing the temperature, you will increase the number of collisions. You will also have a higher number of particles with enough KE to overcome the activation energy barrier. Increasing temperature increases the rate of reaction. CONCENTRATION Increasing the concentration of reactants increases the frequency of collisions. By increasing the concentration, you will increase the number of successful collisions and therefore increase the rate of reaction. PARTICLE SIZE A sugar cube dissolves much more slowly than the same amount of sugar in granulated form. This is because the sugar cube only has its 6 sides in contact with the water; whereas, the granulated sugar has significantly more surface area in contact with the water so dissolving is faster. Decreasing particle size increases the rate of reaction by providing greater surface area which allows for more contact and collisions. PRESSURE OF GASES Increasing the pressure increases the rate of reaction. By increasing the pressure of a gas, you are compressing it into a smaller area which increases its concentration which increases the number of collisions. CATALYST A catalyst increases the rate of both the forward and reverse reactions without undergoing chemical change. A catalyst provides an alternate route for the reaction with a lower activation energy. It is incorrect to say that the catalyst lowers the activation energy. It essentially ensures that without raising the temperature, more particles have sufficient KE to overcome the Ea barrier. UNDERSTANDING/KEY IDEA 6.1.C Concentration changes in a reaction can be followed indirectly by monitoring changes in mass, volume and color. In all of the following techniques, the goal is to measure the change in concentration vs time. Reaction rate is dependent upon temperature so it is crucial to control the temperature. This is best done by a thermostatically controlled water bath. Techniques for measuring rate of reaction Concentrations are not usually measured directly. There is usually some sort of signal which relates to the change in concentration of either the reactants or the products. The raw data collected from these “signals” usually have units other than the units for concentration which is measured in mol/dm3. There are six common techniques which will be discussed. 1. CHANGE IN VOLUME OF GAS PRODUCED If one of your products is a gas, then you can collect the gas and measure the change in volume at regular time intervals. The graph is change in volume vs time. Collection can be done with a gas syringe or by water displacement in a burette. Water displacement is limited if the gas is soluble in water. Warm water should be used since most gases are less soluble in warm water. 2. CHANGE IN MASS If a reaction involves a change in mass, you can measure this directly. The reaction vessel can be placed directly upon a balance and the mass can be recorded against time by being hooked to a data collection set up. The graph would be mass against time. 3. CHANGE IN TRANSMISSION OF LIGHT – COLORIMETRY OR SPECTROPHOTOMETRY If one of the reactants or products is colored, you can measure the change in the color on a colorimeter. Continuous readings can be made of the light transmission. The graph is absorbance vs time. 4. CHANGE IN CONCENTRATION MEASURED USING TITRATION This is not a continuous method because as you are titrating one sample, the rest is still reacting. Samples must be withdrawn at regular time intervals and then analyzed by titration. In order to stop the reaction at each time interval, a substance is introduced to stop the reaction at that moment. This is called quenching. 5. CHANGE IN CONCENTRATION MEASURED USING CONDUCTIVITY Conductivity is a measure of the number of ions in a solution. If a reaction is using up or producing ions, you can measure this by a conductivity meter. 6. NON-CONTINUOUS METHODS OF DETECTING CHANGE DURING A REACTION – CLOCK REACTIONS There are times when you cannot record the continuous change in the rate of a reaction. It may be more convenient to measure the time is takes for a reaction to reach a certain fixed point. This means something you have chosen to use as an indicator to “stop the clock”. The limitation is this method gives only an average rate over the time interval. UNDERSTANDING/KEY IDEA 6.1.D Activation energy (Ea) is the minimum energy that colliding molecules need in order to have successful collisions leading to a reaction. UNDERSTANDING/KEY IDEA 6.1.E By decreasing Ea, a catalyst increases the rate of a chemical reaction, without itself being permanently chemically changed. APPLICATION/SKILLS Sketch and explain energy profiles with and without catalysts. EFFECT OF CATALYST A catalyst increases the rate of the reaction without itself undergoing chemical change. It provides an alternate route for the reaction which has a lower activation energy. Be careful not to say “it lowers the activation energy of the reaction”. APPLICATION/SKILLS Be able to describe the kinetic theory in terms of the movement of particles whose average kinetic energy is proportional to temperature in Kelvin. KINETIC THEORY OF MATTER All matter consists of particles which are in constant motion. The KE for gases is greater than that of liquids which is greater than that of solids. Temperature (measured in Kelvin) is proportional to the average KE of the particles in a substance. The higher the temperature, the higher the kinetic energy. More about kinetics Kinetics refers to movement. Movement in chemistry refers to the progress of a reaction. Therefore, kinetics is the study of how fast the reaction goes. The reaction mechanism is a sequence of bond breaking and bond making which suggests “how” the reaction happens. Even though reaction concentrations decrease, rate is always expressed as a positive number. The graph is a curve so we can only measure the tangent to the curve at a moment in time which will give us the rate at that moment in time. APPLICATION/SKILLS Be able to construct Maxwell – Boltzmann energy distribution curves to account for the probability of successful collisions and factors affecting these, including the effect of a catalyst. Sketch and explain qualitatively the Maxwell-Boltzman energy distribution curve for a fixed amount of gas at different temperatures and its consequences for changes in reaction rate. MAXWELL-BOLTZMANN DISTRIBUTION CURVE The Maxwell-Boltzmann curve shows that particles in a gas at a particular temperature show a range of values of kinetic energy. The area under the curve represents the total particles in a sample. Citations International Baccalaureate Organization. Chemistry Guide, First assessment 2016. Updated 2015. Brown, Catrin, and Mike Ford. Higher Level Chemistry. 2nd ed. N.p.: Pearson Baccalaureate, 2014. Print. Most of the information found in this power point comes directly from this textbook. The power point has been made to directly complement the Higher Level Chemistry textbook by Catrin and Brown and is used for direct instructional purposes only.