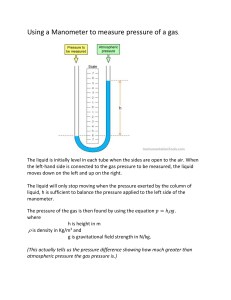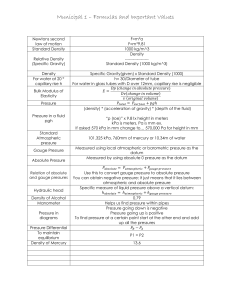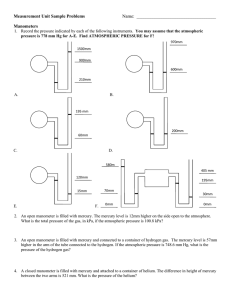
IGCSE Physics – Unit 6: Pressure Unit #6: Pressure 1 [p 83 – 87, 133 – 138] 6.1 Pressure Core Relate, without calculation, pressure to force and area, using appropriate examples. Relate, without calculation, the pressure beneath a liquid surface to depth and to density, using appropriate examples. Extended Recall and use the equation p = F/A. Recall and use the equation p = h g. 6.2 Gas Pressure, Properties & Measurement Core Describe the simple mercury barometer and its use in measuring atmospheric pressure. Use and describe the use of a manometer. Describe qualitatively the pressure of a gas in terms of the motion of its molecules. Describe qualitatively the effect of a change of temperature on the pressure of a gas at constant V. Relate the change in volume of a gas to change in pressure applied to the gas at constant T. Describe qualitatively the effect of a change of temperature on the volume of a gas at constant p. Extended Recall and use the equation pV = constant at constant temperature. 6.1 Pressure [p 83 – 85] Pressure (p) is defined as the force (F) acting per unit area (A). Pressure is a measure of how 'spread out' a force is and the following formula is used to calculate it: F p= A F = force [measured in newton (N)] A = area [measured in metre2 (m2)] 2 p= pressure [measured in newton per square metre (N/m ) or pascal (Pa)] Note: 1 Pa = 1 N/m2 and 1 kPa = 1000 Pa Examples: Stand on the floor on one leg the pressure exerted on the one foot is greater than the pressure exerted when you stand on two feet. Stand on your toes the pressure exerted on your toes is greater than when standing on two feet. Stand on a stone your weightis exerted on a smaller area the pressure exerted is greater. Stand on a drawing pin the area is very small on which the forces is exerted pressure is great pain! ACG Senior College IGCSE Physics – Unit 6: Pressure 1m 2 The box has a mass of 45 kg hence the weight of the box is 450 N. Thus the box exerts a downward force of 450 N. C B 3m 2m The box rests on side A: p = F/A = 450/(3x2) = 450/6 = 75 N/m2 or 75 Pa The box rests on side B: p = F/A = 450/(1x3) = 450/3 =150 N/m2 pr 150 Pa The box rests on side C: p = F/A = 450/(1x2) = 450/2 = 225 N/m2 or 225 Pa The smaller the area on which a force is exerted, the bigger the pressure. An 80 kg man stands on the ground on an area of 0.033 m2. Another 160 kg man stands on the ground on the same area. Compare the pressure exerted by each. p of 80 kg man = F/A = (80 x 10)/0.033 = 24 000 N/m2 or 24 kPa. p of 160 kg man = F/A = (160 x 10)/0.033 = 48 000 N/m2 or 48 kPa. The bigger the force exerted on the same area, the bigger the pressure. In every situation, the amount of pressure exerted is determined by: the size of the force used and the area over which the force is spread. a large force exerted over a small area causes a high pressure a small force exerted over a large area causes a low pressure. Worked examples a snowmobile make if it has a weight of 800 N and the a) What pressure on the snow does 2 runners have an area of 0.2 m ? p = F/A = 800 / 0.2 = 4 000 Pa (4 kPa) b) Find the area that a 30 kg parcel is in contact with the ground, given that it exerts a pressure of 600 Pa. A = F / p = (30 x 10) / 600 = 0.5 m2 Pressure in Fluids (liquids or gases) Pressure in a fluid acts in all directions and is transmitted throughout the fluid. Pressure in the same fluid increases with depth. A can with three equal sized holes in the sides is filled with water. The water from the bottom hole shoots out the farthest because that is where the largest pressure is being exerted. The pressure is largest here due to more water being above it, creating a greater gravity force (weight) pushing on the same area of the can, thus greater pressure. A fluid with greater density will exert a greater pressure than another fluid even when at the same depth. The pressure is larger because there is more mass of the fluid at this depth due to its higher density, creating a greater gravity force pushing on the same area, thus greater pressure. The pressure on a diver at a certain depth depends on: the density of the liquid, the diver's depth, h. ACG Senior College IGCSE Physics – Unit 6: Pressure 3 Change or difference in pressure between any two points of the fluid can be calculated by the following equation: p = h g h g p = = = = depth below the surface of the liquid (in m) density of liquid (in kg/m3) acceleration of free fall (10 m/s2) pressure of the fluid at depth h (in N/m2). Note: The above equation is most commonly used to calculate the pressure below the surface of a liquid which means the p calculated is often just the final pressure at that specific depth. If some sort of pressure already exists at the surface, then the p must be added on to this pressure. Worked example: What pressure is exerted by the water on a diver at a depth of 10 m? of water = 1000 kg/m3 p = h g = 10 x 1000 x 10 = 100 000 Pa = 100 kPa (Note: since the pressure of the water on the diver at the surface = 0, then 0 + 100 = 100 kPa) You should be able to do: 1. What pressure is exerted by: a) a force of 500 N acting on an area of 1.25 m2? b) a force of 360 N acting on a rectangular area of 6 cm by 4 cm? 2. What is the force exerted by a pressure of 30 kPa acting on an area of 40 m2? 3. Calculate the pressure generated by: a) an ordinary shoe heel 5 cm by 5 cm worn by a 40 kg person standing on the heel. 4. b) a 500 kg elephant standing on one foot of 20 cm diameter. c) a 50 kg person standing on a high-heel of area 0.5 cm2. d) Which of the above will damage a wooden floor that starts to yield at a pressure of 4 000 kPa? The pressure exerted by the atmosphere is 100 kPa. What force acts on an area of 25 m2? ACG Senior College IGCSE Physics – Unit 6: Pressure 5. The mass of a car is 1100 kg. What is the pressure exerted on the ground if the area in contact with each tyre is 275 cm2? 6. Explain why a person can lie horizontally on a bed of nails without the nails piercing his skin? 7. Explain: a) 8. 9. why it hurts to hold a heavy parcel by the string. b) why stiletto heels are more likely to mark wooden floors. c) why a sharp knife cuts more easily than a blunt one? A rectangular block of sides 3m by 2m by 1m has a mass of 3000 kg. a) What is the smallest pressure that the block can exert on a flat surface? b) What is the difference between the smallest and the greatest pressure that the block can exert on a flat surface. A car of weight 12 000N stands on level ground. The pressure on each of its four identical tyres is 300 kPa. What is the area of contact between each tyre and the ground? 10. What is the pressure 100 m below the surface of seawater of density 1150 kg/m3? ACG Senior College 4 IGCSE Physics – Unit 6: Pressure 5 11. The pressure gauge on a submarine in a river was reading 100 kPa when it was at the surface. The submarine dived and a sailor noticed that the gauge was then reading 250 kPa. Density of river water = 1000 kg/m3. a) How deep is the submarine? b) How would the answer change if the submarine were diving in seawater that is slightly denser than the fresh river water? 12. The pressure exerted by a column of oil of density 780 kg/m3, is 2 500 Pa. Calculate the length of the column of oil. 13. Explain: a) why the bottom of a dam wall is thicker than the top of the wall. b) how the liquid in a drinking glass is able to move up the straw into a person's mouth. 14. Using the diagram alongside, explain how the tube (with end A under the liquid and end B in the air) can be used to siphon the liquid out of a container. A B ACG Senior College IGCSE Physics – Unit 6: Pressure *15. Read up about how a hydraulic machine works and then try the next two questions (NOT part of IGCSE course, but a very important and useful concept). In a hydraulic press a force of 20 N is applied to a piston of area 0.20 m2. The area of the other piston is 2.0 m2. Determine: a) the pressure transmitted through the liquid b) the force exerted by the other piston? *16. a) b) Why must a liquid and not a gas be used as the fluid in a hydraulic machine? On what other important property of a liquid do hydraulic machines depend? ACG Senior College 6 IGCSE Physics – Unit 6: Pressure 7 6.2 Gas Pressure, Properties & Measurement [p 86, 87, 133 – 138] Gases, like liquids, exert a pressure on any object immersed in them. The pressure is caused by the tiny, rapidly moving gas molecules (400 - 500 m/s) bombarding the object. A gas exerts pressure in ALL directions. A gas exerts pressure because the gas particles are in constant, random motion the gas particles have kinetic energy and collide continuously with the walls of the container they exert a force on the walls of the container. The pressure caused by the air (approx. 20 km high) above the Earth's surface is called atmospheric pressure. Average density of air = 0.5 kg/m3. Consider the height of air 20 km (20 000 m) above an area of 1 m2 on Earth: Hence, atmospheric p = h(column of air)(air)g = 20 000 x 0.5 x 10 = 100 000 Pa = 100 kPa So, at sea level the normal atmospheric pressure is approximately 100 000 Pa (100 kPa). [ on a table of area 1 m2 the air exerts a downward force of 100 000 N (this is equivalent to the weight of 100 people of average mass 100 kg each!) atmospheric pressure at sea level is VERY BIG!!!] Demonstrations of the effect of this big atmospheric pressure: - atmospheric pressure is big enough to crush a can when the air inside is removed (a vacuum) - push liquid up a straw when the air inside the straw is sucked out (pressure inside is lowered) - push two Magdeburg hemispheres together when the air inside is removed so that two teams of horses could not pull them apart as discovered by Otto von Gürcke (mayor of Magdeburg) in 1654 when he invented a vacuum pump - push a column of mercury up to a height of 760 mm in a mercury barometer at sea level. Measuring Pressure Atmospheric pressure can be measured using a barometer: (i) In a mercury barometer atmospheric pressure supports a column of mercury in a tall, sealed tube. The space at the top of the tube is a vacuum (zero pressure). Atmospheric pressure pushes down on the mercury in the reservoir, which in turn pushes mercury up the tube until the pressure of the column of mercury balances the atmospheric pressure. atm. p = h(column)(mercury)g At sea level standard atmospheric pressure is 760 mm of mercury (= 101 300 Pa or 101 300 N/m2 though for simplicity we usually just use 100 000 Pa) (ii) A manometer can be used to compare pressures and is used to measure the pressure difference between two regions. It consists of a U-tube of liquid that is displaced when the pressures at each end of the tube are different. The liquid moves until the pressure difference is balanced by the difference in height of the ends of liquid. The greater the pressure, the greater the difference in height. The pressure difference ( p) is calculated using p = h(column)(liquid)g This is excess pressure because it adds to any p acting on the surface of the liquid in the other arm of the manometer. ACG Senior College IGCSE Physics – Unit 6: Pressure 8 Oil is often used as the liquid in a manometer because: - water evaporates and because - oil (being less dense) makes the manometer more sensitive: for the same pressure difference, the oil will move further. (iii) Atmospheric pressure can be used to predict changes in weather. An aneroid barometer is used for this. If atmospheric pressure increases, the corrugated box is squeezed a little which causes the pointer to move. If the pressure decreases the box expands and the pointer moves the opposite way. Worked example: The manometer on an industrial machine shows the oil is being pushed towards the machine and the height difference, h, is 20 cm. What is the pressure inside the machine if the pressure outside is 100 kPa? The oil has a density of 800 kg/m3. p difference = h(column)(liquid)g = 0.2 x 800 x 10 = 1 600 Pa = 1.6 kPa Pressure inside the machine must be lower than atmospheric pressure, because the "oil is being pushed towards the machine". machine 20 cm Therefore the pressure inside the machine = 100 - 1.6 = 98.4 kPa Pressure Changes in Gases An enclosed (trapped in a container) gas exerts pressure in ALL directions. The gas pressure is the size of the force per unit area of the container walls the greater the number of collisions per second (rate of collisions) with the walls, the greater the pressure. Effect of Temperature on Gas If the piston of a bicycle pump is pushed in and held in the pushed-in position, you can feel the pressure. There is a force trying to push the piston out. If the trapped air inside the pump is heated, the molecules gain kinetic energy and move faster they hit the piston more often and harder the pressure on the piston and the walls of the pump will increase. increasing the temperature of an enclosed gas (constant volume) increases its pressure. Pressure and Volume Relationship of an Enclosed Gas [Boyle's Law] If you push in the piston of a bicycle pump slowly, it becomes harder and harder to do so the more you push it in. This is because the pressure in the pump goes up. There are the same number of air molecules in the pump travelling at the same speed (temperature remains constant). However, because the molecules are now packed into a smaller volume, there will be more collisions per second with the walls and with the piston, and hence the pressure increases. ACG Senior College IGCSE Physics – Unit 6: Pressure 9 If the volume is halved, then the number of collisions per second with the walls and piston will double and hence the pressure will double. Hence, the pressure of an enclosed gas is inversely proportional to the volume, provided the temperature of the gas remains constant. This relationship between pressure and volume was discovered in 1662 by Robert Boyle is known as Boyle's Law. Hence, a fixed amount of gas in a sealed container at constant temperature obeys the following equation: p = pressure of gas (in N/m2 or Pa) V = volume of gas (in m3) p V = constant The constant will be a constant for a particular sample of gas in a particular container. initial values of an experiment p1 V1 = constant and final values of the experiment p2 V2 = same constant value. p1 V1 = constant = p2 V 2 or p1 V1 = p1 V2 [Remember this equation only applies if the temperature remains constant] Worked examples 1. What will be the final pressure of 500 cm3 of air at 100 000 Pa if it is squashed to one fifth of its original volume slowly so that the temperature remains the same? p1 x V1 = p2 x V2 100 000 x 500 = p2 x 100 = (100 000 x 500)/100 = 500 000 Pa (500 kPa) p2 [OR the V decreased by 5 the pressure increases by 5 new p = 5 x 100 000 = 500 000 Pa] 2. A bicycle pump contains 400 cm3 of air at atmospheric pressure. If the air is compressed slowly, keeping the temperature constant, what is the pressure when the volume of the air is compressed to 125 cm5? p1 V1 100 x 400 p2 = p2 V2 = p2 x 125 = 40 000 / 125 = 320 000 Pa = 320 kPa You should be able to do: 1. a) Name three devices that can be used to measure atmospheric pressure. b) Why is a high atmospheric pressure associated with a warm day? ACG Senior College IGCSE Physics – Unit 6: Pressure 2. 10 The diagram alongside shows a simple barometer. a) What is in the space in the tube above the mercury? b) What is the pressure at the top of the column of mercury? 74 cm c) What is the size of the atmospheric pressure as shown by the barometer? 2 cm d) What would happen to this reading if the barometer were taken up a very high mountain Give a reason for your answer. e) Calculate the length of the column of mercury at standard atmospheric pressure of 101 kPa? Density of mercury = 13546 kg/m3. Use g = 9.81 m/s2. 3. Calculate the pressure of the water 100 m below the surface of the sea (density = 1150 kg/m3)? 4. Why is it not a good idea to use water as the fluid in a barometer to measure atmospheric pressure? (Hint: Calculate the length of the column of water at standard atmospheric pressure. Density of water = 1000 kg/m3. 5. A thin-walled plastic bottle is sealed and contains dry air at atmospheric pressure. a) Explain how the molecules of air inside the bottle exert a pressure on the walls. ACG Senior College IGCSE Physics – Unit 6: Pressure 11 b) Ice is placed inside the bottle, and the bottle is then sealed again. The temperature of the air inside the bottle falls and the bottle becomes partially crushed. Explain, in terms of the molecules of air inside and outside the bottle, why this happens. 6. The diagram below shows a water manometer used to measure the pressure inside a gas pipe. Figure 1 Figure 2 Figure 3 a) State whether the pressure inside the gas pipe in Fig. 1 is larger than or smaller than atmospheric pressure. b) The manometers shown in Figs. 2 and 3 are connected to the same gas pipe at the same pressure as shown in Fig. 1. On Figs 2 and 3, draw the levels of the liquid in each manometer if (i) the manometer in Fig. 2 contains water and has tubes with twice the diameter of Fig. 1, (ii) the manometer in Fig. 3 contains a liquid with density half that of water. c) The manometer shown in Fig. 4 has its top end sealed. Fig. 4 Explain why the water levels are different in Figs. 4 and 1, even though the pressure in the gas pipe is the same. ACG Senior College IGCSE Physics – Unit 6: Pressure 7. Use the kinetic molecular model to explain why an aerosol can of underarm deodorant has a large label on the side that says: 'Danger. Do not dispose of the can by throwing it in a fire.' 8. A deep-sea diver is working at a depth where the pressure is 3.0 atmospheres. He is breathing out air bubbles. The volume of each bubble is 2 cm3. At the surface the pressure is 1.0 atmosphere. What is the volume of each bubble when it reaches the surface (assuming its temperature remains the same)? 9. a) A cyclist pushes in the plunger of her bicycle pump slowly, compressing the air to a quarter of its volume. What would be the final pressure if the compression is isothermic (no change in temperature)? b) Describe and explain, in terms of the air particles in the pump, the effect of decreasing the volume on the pressure of the air particles in the pump (temperature is kept constant). c) Describe and explain what happens to the pressure if the air is compressed very quickly? 10. A sealed gas syringe is immersed in a heated water bath. Describe in terms of the gas molecules, why, if the pressure inside the syringe is to remain constant, the gas must expand as its temperature rises. ACG Senior College 12 IGCSE Physics – Unit 6: Pressure 11. a) Describe and explain, in terms of the air molecules, how the pressure changes when the gas in the sealed container is heated. *b) The pressure of a gas in an enclosed container is directly proportional to its absolute temperature (in K). A sealed container in a factory contains a gas at 100 kPa and at a temperature of 280 K. Determine the pressure of the gas if the sealed container is heated to 560 K without exploding. ACG Senior College 13 IGCSE Physics – Unit 6: Pressure Past CIE Exam Questions Multiple Choice questions 1 A student places his thumb firmly on the outlet of a bicycle pump, to stop the air coming out. What happens to the pressure and to the volume of the trapped air as the pump handle is pushed in? 2 Which diagram shows the child exerting least pressure on the ground? 3 A manometer is being used to measure the pressure of the gas inside a tank. A, B, C and D show the manometer at different times. At which time is the gas pressure inside the tank greatest? ACG Senior College 14 IGCSE Physics – Unit 6: Pressure 4 15 The diagram shows an instrument used to measure gas pressure. What is the instrument called? A 5 ammeter B barometer C manometer D thermometer The diagram shows two divers swimming in the sea and two divers swimming in fresh water. Sea water is more dense than fresh water. On which diver is there the greatest pressure? 6 Four flower vases have circular bases. They are filled with water so that they all have the same weight. What vase exerts the greatest pressure on its base? 7 Two sharp nails and two blunt nails are held on a piece of wood. Each nail is hit with the same hammer with the same amount of force. When it is hit, which nail causes the greatest pressure on the wood? ACG Senior College IGCSE Physics – Unit 6: Pressure 8 16 The diagram shows a manometer connected to a container of carbon dioxide. Which statement correctly describes the pressure exerted by the carbon dioxide. 9 A It is equal to the atmospheric pressure. B It is equal to the 5 cm of mercury. C It is equal to 5 cm of mercury above atmospheric pressure. D It is equal to 5 cm of mercury below atmospheric pressure. Liquid X has a density of 1010 kg/m3. Liquid Y has a density of 950 kg/m3. The liquids are poured into tubes as shown. Which tube has the greatest pressure at its base? 10 The pressure at a point in a liquid 1 2 3 increases as the depth increases increases if the density of the liquid increases is greater vertically than horizontally Which statement(s) is (are) correct? A 1, 2, 3 ACG Senior College B 1, 2 C 2, 3 D 1 only IGCSE Physics – Unit 6: Pressure 17 Core questions 1 A garden pot containing soil weights a total of 360 N. The pot rests on three equally-spaced blocks, so that surplus water can drain out of the holes in the base of the pot. The soil is uniformly distributed in the pot. The pot is shown in Fig. 1.1. Fig. 1.1 a) What is the force exerted by each block on the pot? ............................................. N b) State the direction of these forces? ...................................................................................................... c) The gardener finds that the blocks sink into the ground, but he must have the pot up on blocks to allow the drainage. What can he do to reduce the sinking of the pot? ........................................................................................................................................... [3] 2 a) A manufacturer of car tyres estimates that the area of a car tyre in contact with the road is about the same as the area of a person's shoe in contact with the ground. (i) A car weighs 10 000 N and a person weights 500 N. Calculate the ratio pressure of car on ground pressure of person on ground Remember that the car has 4 tyres and a person has 2 feet. (ii) Suggest why it might be a good idea to reduce the pressure of the air in car tyres if the car is to be driven over soft sand or over snow. .............................................................................................................................................. ............................................................................................................................................. .............................................................................................................................................. [5] ACG Senior College IGCSE Physics – Unit 6: Pressure 18 b) A U-tube manometer is used to measure lung pressure by blowing at A, as shown in Fig. 2.1. Fig. 2.1 (i) Before the person blows at A, the liquid levels at X and Y are the same. State the reason for this. ...................................................................................................................................... (ii) Which way do the liquid levels move when the person blows at A? level X moves .............................................. level Y moves .............................................. (iii) What would you measure in order to find the person's lung pressure? .................................................................................................................................... .................................................................................................................................. [4] Extended questions 1 Fig. 1.1 shows a sealed glass syringe that contains air and many very tiny suspended dust particles. Fig. 1.1 (a) Explain why the dust particles are suspended in the air and do not settle to the bottom. ........................................................................................................................................... ........................................................................................................................................... ........................................................................................................................................... .................................................................................................................................... ACG Senior College [3] IGCSE Physics – Unit 6: Pressure 19 (b) The air in the syringe is at a pressure of 2.0 x 105 Pa. The piston is slowly moved into the syringe, keeping the temperature constant, until the volume of the air is reduced from 80 cm3 to 25 cm3. Calculate the final pressure of the air. pressure = ................................................ [3] 2 Explain (a) how air molecules in a sealed box create pressure on the inside walls. ................................................................................................................................................. ................................................................................................................................................. ................................................................................................................................................ (b) why this pressure rises as the temperature of the air in the sealed box increases. ................................................................................................................................................ ............................................................................................................................................... ............................................................................................................................................... [5] Alternative to Practical questions 1 In an experiment to study the effect of increasing pressure on the volume of air, the IGCSE class used the apparatus shown in Fig. 6.1. Fig. 6.1 Fig. 6.2 (a) What is the volume reading shown in Fig. 6.2? volume reading = ........................................ ACG Senior College [1] IGCSE Physics – Unit 6: Pressure 20 Fig. 6.3 Fig. 6.3 shows the graph that one student plotted from the readings. She drew a best-fit curve through the points. Theory suggests that the relationship between pressure and volume is given by equation p x V = constant The student is required to find the value of the constant. (b) Why is it better to find the value of the constant using the graph than from a single measurement of p and V? ........................................................................................................................................... ....................................................................................................................................... [1] ACG Senior College IGCSE Physics – Unit 6: Pressure 21 (c) (i) Use these two examples, taken from the graph, to show that the readings from the experiment support the theory. Example 1: when p = 200 kPa V = ............................... cm3 p V = .................................... Example 2: when V = 37 cm3 p = ............................ kPa p V = ................................... (ii) Using your answers from (c) (i), predict the pressure required to reduce the volume to 18 cm3. p = .................................... kPa [4] ACG Senior College IGCSE Physics – Unit 6: Pressure ACG Senior College 22