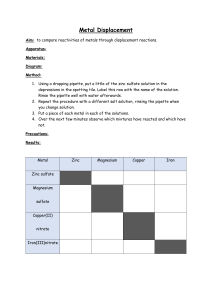
Christian & Missionary Alliance Sun Kei Secondary School 2019 – 2020 S.3 Chemistry Homework - Metal Reactivity Series 26 ) Anthony Li Name: _________________( Date of Submission: 20-2-2020 I Class:S.3_____ 1. A student performs several experiments to determine the order of reactivity of four metals. The results are shown in the table below. Metal Experiment W X Y Z Reaction with cold water reacts readily no observable change no observable change reacts vigorously Reaction with dilute reacts readily reacts very slowly reacts slowly very dangerous NEVER hydrochloric acid attempt (a) Arrange the metals in order of reactivity, starting with the most reactive one. Explain your answer briefly. (4 marks) z w Y X Reactivity: __________ > __________ > __________ > __________ Explanation: ___Z____ is more reactive than __W_____ because ___Z____ reacts with ___cold water vigorously____________ but __W reacts readily_______________. W _______ is more reactive than Y _______ because W _______ reacts with diluted hydrochloric acid fastly react with Y slowly ___________________________________ but ___________________________________. Y _______ is more reactive than X _______ because Y _______ reacts with diluted hydrochloric acid slower the reaction of X is very slow ___________________________________ but ___________________________________. (b) Metal W reacts with oxygen to give a brick-red flame. (i) Suggest what metal W might be. W: (1 mark) Calcium (ii) Write a word equation for the reaction between W and water. metal W+ Water (1 mark) metal W hydrochloric + hydrogen 1920 S3 Chemistry Homework (Metal Reactivity Series) 1 2. The reactivity of metals can be determined by the reaction between metal and acid. Five different metals, calcium, tin, zinc, copper and nickel are added to dilute hydrochloric acid respectively. The gas produced is collected by displacement of water. The height of gas collected in the first 30 seconds in each case is measured. The results are shown in the following table. Metal Height (cm) of gas collected in the first 30 seconds Calcium 5.5 Tin 1 Zinc 4.5 Copper 0 Nickel 2.5 (a) Write a word equation for the reaction between zinc and dilute hydrochloric acid. (1 mark) zinc + hydrochloric acid zinc chloride + hydrogen (b) Suggest a test for the gas evolved. (1 mark) put the lighting split into the gas to test (c) Determine the reactivity series in descending order from the results provided. (1 mark) Ca>zinc>Nickel>Tin>Copper (d) Explain why the reactivity of sodium CANNOT be determined by this method. (1 mark) because sodium will be explosed with diluted hydrochloric acid. 1920 S3 Chemistry Homework (Metal Reactivity Series) 2 2. (e) Another metal was tested and the result was as follows: (i) Metal Height (cm) of gas collected in the first 30 seconds X 5 From the result obtained, rewrite the reactivity series in descending order. (1 mark) Ca>X>Zinc>Nickel>Tin>Mg>copper (ii) Suggest what metal X might be. X: Magnesium (1 mark) 3. X, Y and Z are three different metals. The table below lists the results of three experiments carried out using the metals. Experiment X Y Z Adding metal to cold water no observable change no observable change reacts vigorously Adding metal to dilute hydrochloric acid reacts readily no observable change reacts vigorously Burns in air Bright white light no observable change reacts vigorously (a) Arrange the three metals in order of increasing reactivity. Explain your answer. (Please use the sentence pattern of Q1(a).) (3 marks) Z>X>Y Z is more reactive than X because Z reacts vigorously with lot of hydrochloric acid but X reacts readily.X is more reactive than Y because X reacts with diluted hydrochloric acid readily but Y do not have any observation (b) Suggest what X, Y and Z might be. (3 marks) X: calcium Y: gold Z: potassium 1920 S3 Chemistry Homework (Metal Reactivity Series) 3 4. Answer the following multiple-choice questions. (a) Which of the following is INCORRECT when some sodium granules are put into cold water? A The metal floats on water surface. B An alkaline solution is formed. C The metal moves very rapidly. D The metal burns with a brick-red flame. D Answer: _______ (b) Which of the following combinations describing the reaction between potassium and water is correct? Resultant solution Gas evolved A Acidic Oxygen B Alkaline Oxygen C Acidic Hydrogen D Alkaline Hydrogen D Answer: _______ (c) Which of the following flame colours does NOT match correctly? Metal Flame colour A Magnesium White B Calcium Brick-red C Sodium Blue D Potassium Lilac B Answer: _______ (d) In school laboratory, hydrogen gas can be safely produced by adding dilute hydrochloric acid to A copper. B sodium. C magnesium. D potassium. C Answer: _______ (e) Which of the following metals will NOT form oxide(s) when heated strongly in air? (1) Calcium (2) Platinum (3) Sodium A (1) only B (2) only C (1) and (3) only D (2) and (3) only C Answer: _______ 1920 S3 Chemistry Homework (Metal Reactivity Series) 4