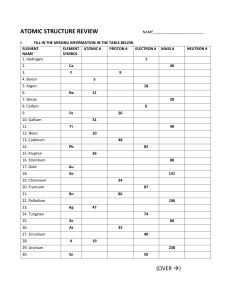
Chemistry Review: Atoms, Elements and Compounds 1. Fill in the blanks with the appropriate terms. a) Two types of pure substances are _____________________ and _____________________. b) The alkali metals have one less electron in the outer electron shell than the ____________. c) In the periodic table, elements in the same ______________________ have similar properties. d) A _______________________ change is a change of state or form. e) ______________________ is the ability of a substance to dissolve in a solvent. f) A chemical change may produce a new solid substance called a ______________________. g) A _________________________ contains only one kind of particle. h) _____________________ are pure substances that contain two or more different elements. i) A(n) _______________________ is a homogeneous mixture of two or more metals. j) The smallest particle of an element is called a(n) _____________________. k) The mass number represents the sum of ________________ and _______________ in an atom. l) _______________________ are elements that have both metallic and non-metallic properties. 2. State three examples of physical properties that would NOT help to identify a pure substance. Explain your answer. 3. What is the difference between a physical property and a chemical property? Give one example of each property to describe oxygen gas. 4. Classify each of the following properties of a cake as qualitative or quantitative. a) It is circular in shape – b) Its mass is 1.5 Kg – c) It tastes like chocolate – d) It is 30 cm in diameter – e) Its icing is melting – 5. Backyard barbecues are fuelled by gas. a) Identify one physical property that barbeque fuel should have. Explain why this property is important for barbeque fuel. b) Identify one chemical property barbeque fuel should have. Explain why this property is important for barbeque fuel. 1 6. What is the mass of a block of wood that measures 2 cm x 5 cm x 1 cm and which has a density of 0.75 g/cm3? 7. Classify each of the following as a physical change or a chemical change. a) frying an egg - e) water freezing on a pond - b) percolating coffee - f) a paper clip bending - c) letting paint dry - g) rust forming on a car - d) toasting bread - h) bleaching a stain - 8. Classify each of the following as an element, compound, solution (homogeneous mixture) or heterogeneous mixture. a) mercury - e) ammonia (NH3) - b) baking soda (NaHCO3)- f) compost - c) detergent - g) Sprite - d) muddy water - h) aluminum - 9. Draw a diagram to represent each of the following: a) A mixture of a diatomic element and a compound. b) A compound. 10. a) Describe the test for oxygen gas. b) How is the test for hydrogen different from the test for oxygen? c) Carbon dioxide will put out a burning splint, but this alone does not confirm the presence of the gas. Why not? 11. What information is presented in a chemical formula? 2 12. Name the elements that are present in each of the following compounds. State the number of atoms of each element that are present for the indicated number of molecules. Formula Type of Number of Each Type of Total Number of Atom Atom Molecules a) 2Mg3(PO4)2 b) Ca3N2 c) 4Na2(SO4) 13. Complete the following table. Standard Atomic Atomic Number Notation # of Protons # of Electrons Atomic Mass # of Neutrons 63 i) 29 Cu ii) 12 12 14 iii) 7N iv) 29 36 ___ v) __Zn 65 14. Draw a Bohr-Rutherford Diagram for the following and complete the standard atomic notation for each. Oxygen Aluminum 15. Explain the difference between a period and a group. 3 16. In the periodic table, where are the metals found? Where are non-metals found? 17. Name two elements that would have similar chemical properties as beryllium. 18. What is meant by the term “valence electron”? How many valence electrons does calcium have? 19. Complete a summary of similarities and differences in the structure and properties among: a) Elements in the same family such as Li, Na, and K. b) Elements in the same period. c) Elements in the family of noble gases. 20. How does the electron structure of noble gases help explain their chemical properties? 21. Explain why an alkali metal would react very vigorously with a halogen gas. 22. Draw a Bohr-Rutherford diagram of a phosphorous atom. Explain what this atom would need to do to become stable. 4