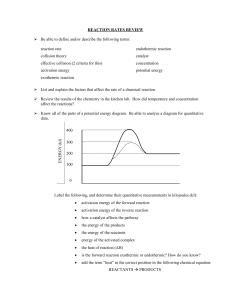
Quiz: Kinetics Chemistry II 1. For each of the following scenarios, describe the effect it will have on the Reaction Rate of any reaction. (a.) An Increase in the Temperature of the System: ____________________ (b.) An Increase in the Concentration of the Reactants: ____________________ (c.) An Increase in the Intermolecular Forces of the Reactants: ____________________ (d.) An Increase in the Intermolecular Forces of the Solvent: ____________________ (e.) An Increase in the Viscosity of the Solvent: ____________________ (f.) An Increase in the Surface Area of a Reactant: ____________________ 2. Will Reaction X have a higher Reaction Rate if its Reactants are in the Same Phase or in Different Phases? ______________________________ 3. Write the Reaction Rate Expression for the reaction aA → bB. 4. For each of the following reactions, determine the Reaction Rate Expression. (a.) 2 N2O5(g) → 4 NO2(g) + O2(g) (b.) 2 SO2(g) + O2(g) → 2 SO3(g) 5. What usually happens the Reaction Rate as time goes on? Why? _____________________________________________________________________________ 6. Given the following reaction and data: (a.) What is the average rate of consumption of NO in the time interval from 10 to 20 seconds? (b.) What is the average rate of production of N2 in that same time interval? 7. For the chemical reaction H2 + Br2 → 2 HBr, what can be said of the relative rates of consumption of the reactants when compared to the formation of product? (A) HBr will be produced at half the rate that bromine is consumed. (B) HBr will be produced at the same rate that bromine is consumed. (C) Br2 and H2 will be consumed at different rates. (D) HBr will be produced at twice the rate that bromine is consumed. (E) It is not possible to tell anything about relative rates without the rate law. 8. Given the reaction: X(g) + 2 Y(g) → XY2(g) If the Rate of Formation of Y(g) is −5.0 × 10−2 mol L−1 sec−1, what is the Rate of Formation of XY2(g)? 9. Given the reaction: CH4 + 2 O2 → 2 CO2 + H2O (a.) Which species has the greatest rate of appearance? ______ (b.) Which species has the greatest rate of disappearance? ______ 10. Write the General Rate Law formula for the reaction A + B + C → D + E. 11. Write the formula used to determine the units of the Rate Constant (k). 12. The rate constant for a gas phase reaction can be increased by doing which of the following? I. Increasing the concentration of reactants II. Increasing the temperature III. Decreasing the size of the container (A) I only (B) II only (C) I and II only (D) I and III only (E) I, II, and III 13. What is the order of the reaction if A decomposes to B and C with a rate constant of 8.43 × 10–4 s–1 at a certain temperature? 14. Given the following reaction and data: (a.) Determine the Rate Law. Clearly show the division of experiments to find the order with respect to each reactant. (b.) Determine the value (with units) of the Rate Constant. (c.) Using the Rate Constant, find the Initial Rate for Experiment 5. 15. Given the following reaction and data: 2W + 3X + Y → Z (a.) Determine the Rate Law. (b.) Determine the Rate of Change of X in Experiment 1. 16. Given the following reaction and data: A + B ⇄ 2C Determine the Experimental Rate Law for the reaction. 17. Given the following reaction and Rate Law: A + B → 2C Rate = k[A][B]3 By what factor does the Rate of Reaction increase when [A] remains constant but [B] is doubled? 18. For a Zeroth Order Reaction: (a.) Draw the resulting Straight-Line Graph on the right. (b.) What is the slope of the line equal to? ______ (c.) Write the Integrated Rate Law equation. (d.) Write the Half-Life formula. 19. For a First Order Reaction: (a.) Draw the resulting Straight-Line Graph on the right. (b.) What is the slope of the line equal to? ______ (c.) Write the Integrated Rate Law equation. (d.) Write the Half-Life formula. 20. For a Second Order Reaction: (a.) Draw the resulting Straight-Line Graph on the right. (b.) What is the slope of the line equal to? ______ (c.) Write the Integrated Rate Law equation. (d.) Write the Half-Life formula. 21. Given the reaction and data: A→B time (s) [A] (M) 0 1.00 5 0.63 10 0.46 15 0.36 20 0.25 (a.) Using you Graphing Calculator, determine the Reaction Order and explain how you figured it out. (b.) Determine the Rate Constant (with units). (c.) Determine the Half-Life of A. (d.) Determine the concentration of A at 300 seconds. 22. Given the following reaction and data: (a.) Using you Graphing Calculator, determine the Reaction Order and explain how you figured it out. (b.) What is the Rate Law? (c.) Determine the Rate Constant. (d.) Determine the Half-Life of the reaction. 23. Given the following data for the reaction A → B: (a.) Using your Graphing Calculator, determine the order of the reaction. (b.) Determine the Half-Life of the reaction. 24. Radioactive Decay reactions are always of what order? ____________________ 25. The radioisotope C-14 (half-life 5730 years) is used for carbon dating. What is the rate constant for the decay of C-14? 26. What is the concentration of A after 50.5 minutes for the reaction A → B when the initial concentration of A is 0.250 M? (k = 0.117 M–1 min–1) 27. The initial concentration in a reaction is 0.75 M. The rate constant k is 0.015 M/min (a.) What will be the concentration of the reactant after 15 minutes? (b.) How long will it take the concentration to be reduced to 0.06 M? 28. The conversion of cyclopropane to propene is a reaction with a rate constant of 6.7 × 10–4 s–1 at 500 ºC. (a.) If the initial concentration of cyclopropane is 0.0500 M, what is the concentration of cyclopropane after 30 minutes? (b.) How long does it take for the concentration of cyclopropane to reach 0.0100 M? 29. What is Activation Energy? _____________________________________________________________________________ 30. What effect will increasing the Activation Energy of a reaction have on the Reaction Rate? ______________________________ 31. Collision theory predicts all of the following EXCEPT that (A) a reaction will only occur if the collision geometry is correct (B) a reaction will not occur if the collision occurs with energy that is less than the activation energy (C) a reaction will not occur if the reactants do not collide (D) frequency of collisions will increase with increasing temperature (E) more successful collisions will occur for a reaction with a larger activation energy 32. Write the Formula for ∆H (∆E): ∆H =_______________________________ 33. For an Exothermic reaction: (a.) What is the Sign of ∆H (∆E)? _____ (b.) Sketch a Potential Energy Diagram (Label ∆H) 34. For an Endothermic reaction: (a.) What is the Sign of ∆H (∆E)? _____ (b.) Sketch a Potential Energy Diagram (Label ∆H) 35. Explain how a Catalyst effects a reaction. _____________________________________________________________________________ 36. What effect does a Catalyst have on the Activation Energy of a reaction? ______________________________ 37. Draw a Potential Energy Diagram (Label the Activation Energy and the Activated Complex / Transition State). Then, draw a dotted line showing an updated path for the reaction due to the addition of a catalyst (Label the Updated Activation Energy and the Updated Activated Complex / Transition State). 38. Which pair of potential energy/reaction coordinate diagrams represents a comparison between the same reactants where one of the pair of diagrams represents a situation that includes a catalyst? 39. Which pair of potential energy/reaction coordinate diagrams represents a comparison between a reaction with high activation energy to a reaction with lower activation energy but the same heat of reaction? 40. What’s the difference between a Heterogeneous Catalyst and a Homogeneous Catalyst? _____________________________________________________________________________ 41. Does the Arrhenius Frequency Factor of a reaction Change if Temperature Changes? ________________________________________ 42. What is the value (and units) of the Gas Constant needed for the Arrhenius Equation? 43. True or False: Increasing Temperature Increases the Number of Collisions 44. Write the Arrhenius Equation. 45. Write the Arrhenius Equation in its Logarithmic Form. 46. Given the following reaction and data: NO2(g) + O3(g) → NO3(g) + O2(g) Calculate the Activation Energy. 47. An experimental plot of ln(k) vs. 1/T is obtained in lab for a reaction. The slope of the best-fit line for the graph is –4755 K. What is the value of the activation energy for the reaction in kJ/mol? 48. Match each definition/description with one of the choices. (1) Reaction Mechanism (2) Catalyst (3) Rate-Determining Step (4) Intermediate (a.) ____ Describes the series of steps that a chemical reaction is broken in to (b.) ____ Is present at both the start and the end of a reaction (c.) ____ The specific part of a chemical reaction that can be used to determine the rate equation (d.) ____ Isn’t present at the start nor the the end of a reaction 49. Given the following elementary steps for a reaction and their rate constants: (Units of k are not shown) Step 1: Step 2: H2 + ICl → HI + HCl HI + ICl → I2 + HCl k = 0.0005 k = 0.38 (a.) Determine the molecularity for each elementary step. Step 1: ____________________ Step 2: ____________________ Step 3: ____________________ (b.) Which step is the Rate Determining Step? Why? ____________________________________________________________ (c.) If this reaction is Exothermic, Sketch a Potential Energy Diagram for this reaction. 50. Determine the Rate Law for the reaction, 2 NO(g) + Cl (g) → 2 NOCl (g), which has the following Mechanism: Step 1: NO (g) + Cl (g) → NOCl (g) Step 2: NOCl (g)+NO(g) → 2NOCl(g) k ≈ 1 x 10–10 M–1 s–1 k ≈1 x 104 M–1 s–1 51. The following series of reactions show the catalyzed conversion of oxygen to ozone: (a.) Determine the overall reaction. (b.) Determine any Catalysts and any Intermediates Catalysts: ____________________ Intermediates: ____________________ 52. True of False: Catalysts May Appear in the Rate Law 53. True of False: Intermediates May Appear in the Rate Law 54. Given the following elementary steps for a reaction: Step 1: 2 NO + N2O2 ⇄ N2O2 (fast) Step 2: Step 3: N2O2 + H2 → H2O + N2O N2O + H2 → N2 + H2O (slow) (fast) (a.) Determine the molecularity for each elementary step. Step 1: ____________________ Step 2: ____________________ Step 3: ____________________ (b.) Determine the overall reaction. (c.) List any intermediates and any catalysts. Intermediates: ______________________________ Catalysts: ______________________________ (d.) Which step is the rate determining step of the overall reaction? Why? ________________________________________________________________________ (e.) Determine the Rate Law for the overall reaction. (f.) What is the overall reaction order? ____________________ (g.) What are the units of the rate constant?