Reading Guide Chapter 14
advertisement

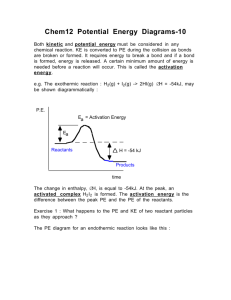
AP Chemistry Chapter 14 Reading Guide 1. What is someone who is studying kinetics trying to figure out? 2. If reaction rates are listed as positive numbers, why is the rate of phenolphthalein disappearing reported as negative? 3. Complete exercise 14.1 (cover up the answers) and check your work. 4. What is a rate law and what is a rate constant? 5. What is the connection between the rate law and the stoichiometry of a chemical reaction? 6. What is meant by the terms unimolecular and bimolecular? 7. What is the order of a reaction? 8. What is meant by zero, first and second order reactions? 9. What is meant by the overall order of a reaction? 10. Summarize collision theory. 11. What is a rate-limiting step? (Beware, the textbook makes it sound like it is always the first reaction, and it does not have to be. They are speaking specifically about the example, only.) 12. What is a reaction mechanism? 13. What is the connection between the overall rate law and the rate limiting step? 14. What is an intermediate? (Be very specific, as they demand you are on the AP test.) 15. Summarize how a reaction can be zero order in respect to all the reactants. 16. In section 14.11 and exercise 14.6 the textbook solves for the order a reaction in a very mathematically intensive way. Although this is not the way we will do it in class, is it correct, and will ALWAYS give you the correct results, even with fractional orders (yes, one year there was a fractional order on the AP test). Give this section a good reading and be sure you understand what this technique accomplishes, but do not be afraid – there are other less mathematically intensive methods that work most of the time. 17. What does every symbol stand for in the three integrated rate laws? 18. If all radioactive decay processes are first-order, why does it also follow first order kinetics? 19. What is a half-life? 20. Table 14.3 is an important summary of the integrated rate laws and half-life equations for each. Compare the chart with the formulas provided on the AP exam (given to you at the beginning of the year). Which equations are provided (and thus could be required of you to calculate). As they are not labeled on the AP exam formula chart, determine a way for you to memorize which equation works for which order/situation. 21. Consider you have graphs like figures 14.11, 14.12, and 14.13 for each of the orders – zero, first, and second. a. Which graph would have a straight line for each order? b. What is the relationship between the rate constant, k, and the slope of the graph? 22. Often in lab we will have reactions that are second order overall (first order in each of two reactants), which is very difficult to analyze. To make it easy we set up the reaction to be pseudo-first order. Summarize how this is accomplished. 23. What is activation energy? 24. What is the typical effect of temperature on activation energy? Reference figure 14.16 in your answer. Be sure to explain both increasing and decreasing temperature. 25. What are the criteria for something to be classified as a catalyst? 26. What is the typical effect of a catalyst on activation energy? Reference both figures 14.17 and 14.18 in your answer.