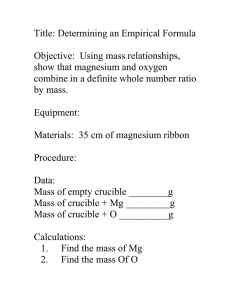
Empirical & Molecular Formula DETERMINING THE FORMULA OF A COMPOUND Empirical formula: the simplest whole-number ratio of atoms in a compound ● It is determined through experiments, empirically. ● Ex: CH, CH4, CH2O, K2Cr2O7. Molecular formula: the actual ratio of atoms in a compound ● It is always a whole number multiple of the empirical formula. ● Ex: C6H6, CH4, C6H12O6, K2Cr2O7. ** The empirical and molecular formula can be the same. Empirical Formula Molecular Formula C6H6 MgCl2 C6H12O6 PERCENT COMPOSITION OF COMPOUNDS The percent composition of a compound can be described by the number of atoms or by percent mass. The percent mass tells the chemist how much of each element or each polyatomic ion is present in a compound on a percent basis. Percent mass of each element in a compound: 1. Calculate the molar mass of the compound. 2. Calculate how much of the molar mass comes from each element. 3. Divide each element’s mass contribution by the total molar mass and multiply by 100 to convert to percent. Percent mass of element A in molecule X= no. of atoms of A x Atomic mass of A x 100 Molar mass of X Empirical & Molecular Formula Example 1 (Percent Composition): Calculate the percent mass of hydrogen in ethanol, C2H5OH. Mr C2H5OH = (12.01 x 2) + (1.01 x 6) + (16.00 x 1) = 46.08 Percent mass of H in C2H5OH = 1.01 x 6 x 100 = 13.15% 46.08 Example 2 (Percent Composition): Calculate the percent mass of each element in glucose, C6H12O6. Ans. 39.99% C; 6.73% H; 53.28% O Empirical & Molecular Formula A Simple Rhyme for A Simple Formula Poem by Joel S. Thompson Percent to Mass Mass to Mole Divide by Small Multiply ‘til Whole Solving for Simple Formulas: a. PERCENT TO MASS: Assume the compound has a mass of 100 grams. You can, therefore, convert the percentage to grams. b. MASS TO MOLES: Calculate the moles of each kind of atom present. c. DIVIDE BY SMALL: Determine the simplest whole number ratios by dividing the moles of each compound by the smallest calculated mole value. d. MULTIPLE ‘TIL WHOLE: If the numbers obtained in the previous step are not whole numbers, multiply each number by an integer so that the results are whole numbers. (ex: numbers such as 9.92 and 1.08 should be rounded, but numbers such as 2.25, 4.33, and 2.72 should not be rounded). e. When writing empirical formulas, the most metallic element (least electronegative) is written first. For organic compounds, write C, H, O than any other element. 5. To determine the molecular formula: Molar mass of molecular formula = number Molar mass of empirical formula Multiply each subscript in the empirical formula by this number to obtain the molecular formula. Empirical & Molecular Formula Example 1 (Simple Formula): Determine the empirical and molecular formula for a compound that gives the following analysis (in percent mass): 71.65% Cl, 24.27% C, and 4.07% H. The molar mass is known to be 98.96 g/mol. 71.65 g Cl · 1 mol Cl = 2.021 mol Cl = 1X 35.45 g Cl 2.021 mol 24.27 g C · 1 mol C = 2.021 mol C = 1X 12.01 g C 2.021 mol 4.07 g H · 1 mol H = 4.0297 mol H = 2X 1.01 g H 2.021 mol empirical formula: CH2Cl molecular mass = 98.96 = empirical mass (12.01 x 1) + (1.01 x 2) + (35.45 x 1) 98.96 = 2 49.48 molecular formula = (CH2Cl)2 = C2H4Cl2 Example 2 (Simple Formula): 2.476 g of an oxide of copper is found to contain 2.199 g copper. Determine its empirical formula. Empirical & Molecular Formula Find the empirical formula for a hydrocarbon compound when given the mass of carbon dioxide and water. CxHy + O2(g) excess → xCO2(g) + y 2 H2O(g) 1. Find the moles of carbon in the compound: ● Find the number of moles of carbon dioxide ● Convert these moles to moles of carbon- 1C: 1CO2 ratio (x:x ratio) 2. Find the moles of hydrogen in the compound: ● Find the number of moles of water ● Convert these moles to mole of hydrogen- 1H2O: 2H ratio ( 3. Follow the same steps as before Percent to Mass Mass to Mole Divide by Small Multiply ‘til Whole y 2 : y ratio) Example 1 (Hydrocarbons): 2.80 g of an organic compound, containing only carbon and hydrogen forms 8.80 g of carbon dioxide and 3.60 g of water when it undergoes complete combustion. Determine its empirical formula. Example 2 (Hydrocarbons): A hydrocarbon contains 92.24% by mass of carbon and its Mr= 78.1. Determine its molecular formula. Empirical & Molecular Formula Find the empirical formula for a CHO compound when given the mass of carbon dioxide and water and the compound. Mass of O = mass of compound – mass of C – mass of H Find the empirical formula for a CHO compound when given the mass of carbon dioxide and water. CxHyOz + O2(g) excess → xCO2(g) + y 2 H2O(g) 1. Find the moles of carbon in the compound: ● Find the number of moles of carbon dioxide ● Convert these moles to moles of carbon- 1C: 1CO2 ratio (x:x ratio) 2. Find the moles of hydrogen in the compound: ● Find the number of moles of water ● Convert these moles to mole of hydrogen- 1H2O: 2H ratio ( 2y : y ratio) 3. Calculate the mass of carbon and hydrogen in the compound by multiplying by their molar mass. 4. Calculate the mass of oxygen and then the moles of oxygen directly. 5. Follow the same steps as before Divide by Small Multiply ‘til Whole Example 1 (C, H, O): Consider vitamin C, a compound that contains carbon, hydrogen, and oxygen only. On combustion of 1.00g vitamin C, 1.50 g CO2 and 0.408 g H2O are produced. Determine the empirical formula of vitamin C. Example 2 (C, H, O): A compound contains only carbon, hydrogen, and oxygen. Combustion of 10.68 g of the compound yields 16.01 g CO2 and 4.37 g H2O. The molar mass of the compound is 176.1 g mol-1. What are the empirical and molecular formulas of the compound? Empirical & Molecular Formula