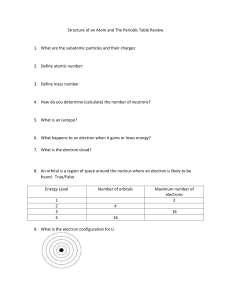
Name: _________________________________ Period: _____ Fall Semester Review Directions: Watch the video (until 4:08) as a review. Reference your color coded periodic table and previous worksheets to help you answer the following questions. Part I: Periodic Table Organization 1. Label the shaded regions of the periodic table below as metals, nonmetals and metalloids (semimetals) 2. Label the groups on the periodic table below as: Noble gases, Halogens, Alkali Metals, Alkaline Earth Metals, Transition Metals, Lanthanides and Actinides 3. Use your periodic table to identify the element based on its described location a. Halogens, period 4 ___________________ b. Alkali metals, period 2 ___________________ c. Noble gases, period 5 ___________________ d. Alkaline earth metals, period 3 ___________________ e. Transition metal, four unpaired electrons ___________________ f. A full third energy level ___________________ g. A metalloid in the second energy level ___________________ Name: _________________________________ Period: _____ Part II: Electrons 4. Circle the correct charge for the following subatomic particles a. Electrons have a (positive/negative/neutral) charge b. Neutrons have a (positive/negative/neutral) charge c. Protons have a (positive/negative/neutral) charge 5. Complete the table below, you may use shorthand/noble gas notation. Element Electron Configuration Valence Electrons a. Fluorine b. Magnesium c. Arsenic d. Zirconium 6. Determine the number of valence electrons for the following elements: a. Beryllium _____ e. Oxygen _____ b. Carbon _____ f. Calcium _____ c. Gallium _____ g. Sodium _____ d. Krypton _____ h. Phosphorus _____ 7. Complete the table below using the ions provided: Ion Circle one Circle one a. Cl- gained/lost electron(s) cation/anion b. O2- gained/lost electron(s) cation/anion c. Mg2+ gained/lost electron(s) cation/anion d. Al3+ gained/lost electron(s) cation/anion e. Br- gained/lost electron(s) cation/anion Electron Configuration