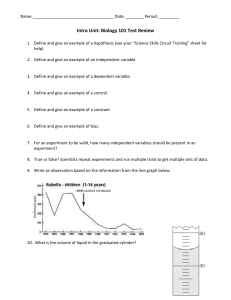
DENSITY WORKSHEET Name:__________________________ Class:____________ Question 1 1.1 A block of aluminium occupies a volume of 15.0 𝑚𝑙 and weighs 40.5 𝑔. What is its density? 1.2 (2) Mercury metal is poured into a graduated cylinder that holds exactly 22.5𝑚𝑙. The mercury used to fill the cylinder weighs 306.0 𝑔. From this information, calculate the density of mercury. 1.3 (2) What volume of silver metal will weigh exactly 2,5 kg. The density of silver is 10.5 𝑔. 𝑐𝑚−3 . (2) 1.4 The figure below gives the dimensions of a rectangular prism of gold. Using this information, calculate the density of gold if the prism has a mass of 1391𝑔. (4) 1.5 A cylinder has a radius of 15𝑐𝑚 and a height of 50𝑐𝑚. Calculate the mass of distilled water that would be required to fill this container. Conversion 1 𝑚𝑙 = 1𝑐𝑚3 Density planning Task 1: measure volume using cylinder (5) DENSITY PRACTICAL GRADE 8 CORNWALLHILL COLLEGE 1. Finding Density You are provided with: - 1 Graduated cylinder 3 cubes (iron, wood, aluminium) Triple beam balance Water Ruler Use the given apparatus to determine the density of the 3 blocks in two different ways. Record your findings in the table below. Block Mass (𝒈) Volume using Graduated Cylinder (𝒎𝒍) Volume using ruler measurement (𝑽 = 𝒔𝟑 )(𝒄𝒎𝟑 ) Density 𝒎 (𝒅 = 𝒗 ) (𝒈. 𝒄𝒎−𝟑 ) Wood Iron Aluminium Using your information above, arrange the blocks in order of increasing density. 2. Make your own Lava Lamp You are provided with: - A 300ml beaker Oil Water Food colouring Medicine dropper Eno effervescing tablet Method 1. 2. 3. 4. Fill the beaker with 200ml of oil. . Add another 100ml of water. Use medicine dropper to drop a few drops of food colouring into your mixture. Now, place one Eno effervescing tablet into your beaker and watch the magic happen.