Quiz 10
advertisement
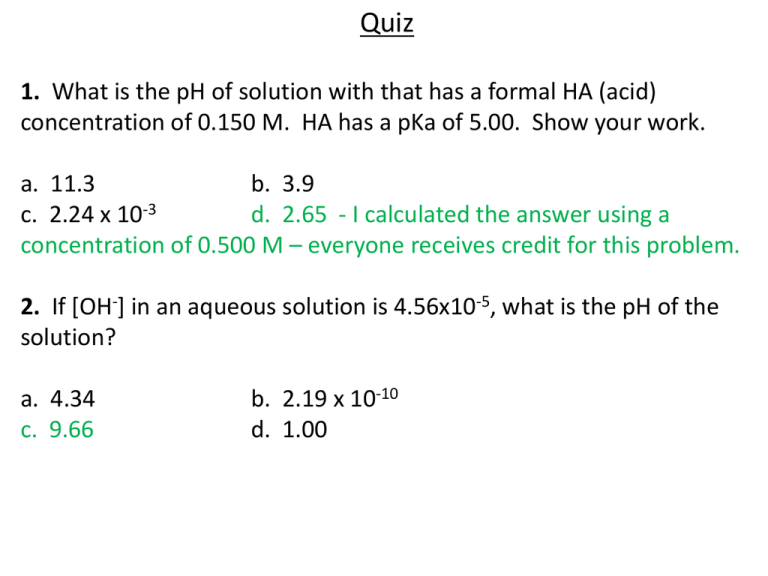
Quiz 1. What is the pH of solution with that has a formal HA (acid) concentration of 0.150 M. HA has a pKa of 5.00. Show your work. a. 11.3 b. 3.9 c. 2.24 x 10-3 d. 2.65 - I calculated the answer using a concentration of 0.500 M – everyone receives credit for this problem. 2. If [OH-] in an aqueous solution is 4.56x10-5, what is the pH of the solution? a. 4.34 c. 9.66 b. 2.19 x 10-10 d. 1.00 Quiz 3. What is the pH of a 3.5 x 10-4 M solution of acetate ion? a. b. c. d. 3.5 10.5 2.9 x 10-11 Need additional information to solve the problem. 4. How much more OH- ion is in a solution with a pH of 4 versus a solution with a pH of 2? a. b. c. d. double the amount 10 times more 100 times more 1000 times more











