Writing Binary Ionic Formulas

Name__________________________________________ Per _______ Date _______________________
7.1 & 7.2 Practice Worksheet B
Writing Binary Ionic Formulas
Part 1: Ion Charges
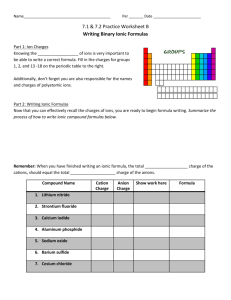
Knowing the __________________ of ions is very important to be able to write a correct formula. Fill in the charges for groups
1, 2, and 13 -18 on the periodic table to the right.
Additionally, don’t forget you are also responsible for the names and charges of polyatomic ions.
Part 2: Writing Ionic Formulas
Now that you can effectively recall the charges of ions, you are ready to begin formula writing. Summarize the three steps of the criss-cross method below.
1.
2.
3.
Remember: When you have finished writing an ionic formula, the total ___________________ charge of the cations, should equal the total ____________________ charge of the anions.
Compound Name Formula
1.
Lithium nitride
Cation
Charge
Anion
Charge
Show work here
2.
Strontium fluoride
3.
Calcium iodide
4.
Aluminum phosphide
5.
Sodium oxide
6.
Barium sulfide
7.
Cesium chloride
Part 3: Writing Formulas with Ions of more than one charge
Practice the following examples. Be careful! These examples contain elements with more than one charge.
1.
Compound Name
Copper (I) fluoride
Cation
Charge
Anion
Charge
Show work here
Formula
2.
Copper (II) fluoride
3.
Tin (IV) chloride
4.
Cobalt (II) phosphide
5.
Manganese (III) phosphide
6.
Iron (III) oxide
7.
Iron (III) sulfate
Part 4: Writing Formulas with Polyatomic Ions
Watch out! This next set of elements contains some of the hardest examples I could give because they may contain polyatomic ions and metals with more than one charge.
Compound Name Formula
1.
Copper (I) hydroxide
Cation
Charge
Anion
Charge
Show work here
2.
Mercury (I) hydroxide
3.
Tin (II) sulfate
4.
Calcium bicarbonate
5.
Manganese (III) phosphate
6.
Gold (I) dichromate
7.
lead (IV) cyanide