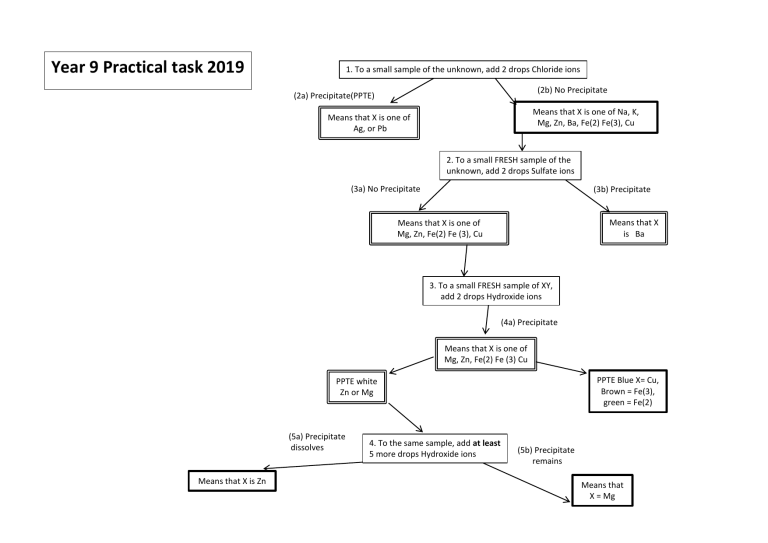
Year 9 Practical task 2019 1. To a small sample of the unknown, add 2 drops Chloride ions (2b) No Precipitate (2a) Precipitate(PPTE) Means that X is one of Na, K, Mg, Zn, Ba, Fe(2) Fe(3), Cu Means that X is one of Ag, or Pb 2. To a small FRESH sample of the unknown, add 2 drops Sulfate ions (3a) No Precipitate (3b) Precipitate Means that X is Ba Means that X is one of Mg, Zn, Fe(2) Fe (3), Cu 3. To a small FRESH sample of XY, add 2 drops Hydroxide ions (4a) Precipitate Means that X is one of Mg, Zn, Fe(2) Fe (3) Cu PPTE Blue X= Cu, Brown = Fe(3), green = Fe(2) PPTE white Zn or Mg (5a) Precipitate dissolves Means that X is Zn 4. To the same sample, add at least 5 more drops Hydroxide ions (5b) Precipitate remains Means that X = Mg