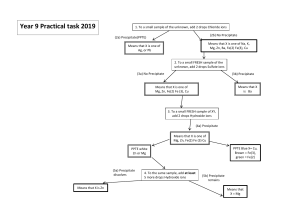
Chem 2 Exp 7 Qualitative Analysis Pre-laboratory Assignment 1. Read in Brown, LeMay, Bursten, Murphy, and Woodward, Section 17.7 Qualitative Analysis for Metallic Elements. 2. Read through all of the procedures and review the flowcharts shown in Figure 1 and Figure 2. Using the flowcharts and the procedures, design a complete flowchart (similar to the flowchart shown in Figure 2) showing the procedures and chemical formulas for each cation. NOTE #1: The flowchart must be done accurately and thoroughly before entering the laboratory. It will not be possible to perform this experiment without a completed flowchart. NOTE #2: Wear gloves and safety goggles. Chemicals Needed 6 M HCl (hydrochloric acid 6 M H2SO4 (sulfuric acid) 6 M HNO3 (nitric acid) 6 M acetic acid 6 M NH3 (aq) (aqueous ammonia) 6 M NaOH (sodium hydroxide) 1 M K2CrO4 (potassium chromate) 1 M K2C2O4 (potassium oxalate) 0.2 M KSCN (potassium thiocyanate) 0.2 M Na3PO4 (sodium phosphate) 1% dimethylglyoxime Equipment and Supplies Needed (1) 100-mL beaker (for hot water bath) (2) 12-mL centrifuge tubes (1) centrifuge (16) 13x10-mm test tubes (1) test tube brush with narrow tip (1) test tube rack (2) plastic transfer pipet (1) glass stirring rod (1) spatula with narrow tip (1) evaporating dish (1) watch glass (1) pH paper vial (1) hot plate Figure 1 Flowchart for Qualitative Analysis (Cation1)n+, (Cation2)n+, (Cation3)n+, (Cation4)n+, (Cation5)n+ (Cation1)n+ + Cl- (Cation1)Cln(s) HCl (aq) Soluble Insoluble (Cation1)Cln (s) (Cation2)n+, (Cation3)n+, (Cation4)n+, (Cation5)n+ GROUP I Cations (Cation2)n+ + S2- (Cation2)2Sn (s) H2S, HCl (aq) Insoluble Soluble (Cation2)2Sn (s) GROUP II Cations (Cation3)n+, (Cation3)n+ + HO- (Cation4)n+, (Cation5)n+ (Cation3)OHn (s) Insoluble (Cation3)OHn (s) NaOH (aq) Soluble (Cation4)n+, (Cation5)n+ GROUP III Cations (Cation4)n+ + PO43- (Cation4)3(PO4)n (s) Insoluble (Cation4)3(PO4)n (s) GROUP IV Cations Na3PO4 (aq) Soluble (Cation5)n+ (aq) GROUP V Cations Introduction Qualitative analysis is the detection and identification of specific chemical components of an unknown sample. This kind of analysis has important applications as diverse as determining blood chemistry abnormalities in an ill person to analysis of materials used in computer circuit assembly. The kind of analysis required for a particular application depends on the likely components of the unknown sample. In this experiment, an unknown sample containing a mixture of inorganic salts will be analyzed. The mixture may contain any of the cations listed below: Ag+, Ba2+, Ca2+, Fe3+, NH4+, Ni2+, Pb2+ Cu2+ maybe present in the solution but will not be analyzed in this laboratory experiment. 2 The cations can be separated into five major groups (Group I cations, Group II cations, Group III cations, Group IV cations, and Group V cations), by differences in the solubilities of their salts. Specific tests, which are unique for each cation, will be performed. Using the flowcharts and the experimental procedures provided, each student will follow a scheme for the qualitative analysis of the mixture of cations. As a positive control for the analysis, a known mixture of cations, will be tested in parallel with the unknown mixture. The following experimental procedures will be performed for the corresponding group of cations. PROCEDURE G-1 test Separation and Analysis of Group I Ions Group I ions are separated from the other cations in the solution by the addition of HCl. The Group I metal chlorides precipitate from the solution and are separated from the remainder of the solution (which contains the dissolved Group II, III, IV, and V ions). Analyze both a sample of the known reference solution and the unknown solution. Measure 1 mL of both the known and unknown solutions into separate centrifuge tubes. To both centrifuge tubes, add 6 drops of 6 M HCl. Centrifuge the samples. After centrifugation, the precipitate should be settled on the bottom of the centrifuge tube and the supernatant (the liquid) should be clear. To test for complete precipitation of the Group I ions, add 1 drop of 6 M HCl to the clear supernatant. If the supernatant turns cloudy, then precipitation is incomplete. If precipitation is incomplete, add 2 drops of 6 M HCl, stir, and centrifuge. Repeat until no more precipitate forms. (It is imperative that the Group I ions be completely removed in this step.) Carefully, pipet the liquid into a clean test tube. The supernatant contains the Group II, III, IV, and V ions (label and save for additional analyses). Wash the precipitate with 5 drops of cold, deionized water (discard the wash water). Predict which ions are in the precipitate. Perform additional tests to confirm the presence of Group I ions. G-2 test Separation and Analysis of Group II Ions The Group II ions can be separated from the Group III, IV, and V ions by using the precipitating agent hydrogen sulfide (H2S) in acidic solution. The Group II ions precipitate as sulfides on reaction with S2+ from H2S. We will not analyze any Group II ions. Therefore, the G-2 test will be skipped and we will move to the G-3 test. 3 G-3 test Separation and Analysis of Group III Ions The Group III ions form sulfides that are soluble in acidic medium, but they may be precipitated if the medium is changed to basic. Group III cations also form insoluble hydroxides. In order to avoid using hydrogen sulfide gas, we will separate Group III ions as hydroxides rather than sulfides. To the supernatant produced from the G-1 test, add ~15 drops of 6 M NaOH dropwise until the solution is basic (test with pH paper after thoroughly mixing the reaction mixture). Centrifuge and save the supernatant for the Group IV and V ion tests (label and save for additional analyses). Wash the precipitate with 10 drops of distilled water, centrifuge, and remove the wash. Decide which additional tests must be performed to confirm the presence of the Group III ion(s). G-4 test Separation and Analysis of Group IV Ions The ions in Group IV can be separated from the Group V ions by addition of sodium phosphate (Na3PO4). To the supernatant from the G-3 test, add 10 drops of 0.5 M Na3PO4. Centrifuge. Save the supernatant for the Group V ion tests (label and save for additional analyses) and save the precipitate for the Group IV ion tests. Decide which ions are remaining in the solution and which additional tests must be performed to confirm the presence of the Group IV ion(s). G-5 test Separation and Analysis of Group V Ions The ions in Group V do not precipitate from solution because their corresponding chlorides, sulfides, hydroxides, and phosphates are reasonably soluble. Decide which ions are remaining in the solution and which additional tests must be performed to confirm the presence of the Group V ions(s). 4 Figure 2 Flowchart of Tests for Each Cation Ag+, Ba2+, Ca2+, Fe3+, NH4+, Ni2+, Pb2+ (fill-in) test HCl (aq) precipitate supernatant GROUP I cations Chemical formula? (s) G-2 test (s) supernatant (fill-in) Hot H2O test (aq) (aq) supernatant solid Chemical formula? GROUP III, IV, & V cations Group II ions test will not be performed (aq) Which (aq) cation? (s) K2CrO4 (aq) test (fill-in) precipitate (aq) NaOH (aq) test (fill-in) Chemical (s) formula? 1. NH3 (aq) 2. HNO3 (aq) (aq) supernatant precipitate GROUP IV and V cations (aq) (aq) (aq) supernatant discard (s) Chemical formula? Chemical formula? GROUP III cations (s) (s) test (fill-in) HCl (aq) Note: Use original sample for this analysis. precipitate test (fill-in) NaOH (aq) test (fill-in) divide solution into two test tubes 1. NH3 (aq) 2. Dimethylglyoxime Confirms which cation? Chemical formula? test (fill-in) Confirms which cation? Na3PO4 (aq) test (fill-in) (aq) KSCN (aq) Confirms which cation? precipitate Chemical formula? (s) H2SO4 (aq) Chemical formula? 1. Acetic acid 2. K2CrO4 (aq) supernatant 1. NH3 (aq) 2. K2C2O4 (aq) test (fill-in) Confirms which cation? (s) 5 Tests for Specific Cations A-1 test Confirmation of Ammonium ions (NH4+) The ions still remaining in solution after the Group I-IV ions have been removed from the solution are the Group V ions. Virtually all ammonium salts are soluble in water. In addition to ammonium salts being virtually inert, the ubiquitous nature of ammonium salts makes the analysis extremely difficult. To improve the chances of no contamination of an external ammonium source, the test for ammonium ions must be performed on the original sample of both the known reference solution and the unknown solution. A litmus test can be used to analyze for the presence of NH4+. Place 2 mL of the original sample in an evaporating dish and add 2 mL of 6 M NaOH. Moisten the strip of red litmus paper or full-range pH paper and stick it to the convex side of a watch glass. Cover the dish with the watch glass (convex side down) making sure that none of the solution in the dish touches the litmus paper. Warm the dish gently using a hot plate (avoid splashing, do not boil). Allow the dish to sit for a few minutes while watching the litmus paper. A change in color to basic is due to the release of NH3(g), which confirms the presence of NH4+. B-1 test Confirmation of Barium ions (Ba2+) Obtain the centrifuge tube or test tube containing the Group IV ions and add 10 drops of 6 M acetic acid to dissolve the solid. Next, add 3 drops of 1 M K2CrO4. The formation of a yellow precipitate, BaCrO4, indicates the presence of Ba2+. Centrifuge the mixture, then pipet the supernatant into a test tube labeled C-1 (for additional Group IV ion tests.) The precipitate can be used for additional tests to confirm the presence of Ba2+. An additional confirmation of the presence of Ba2+ can be obtained by adding 10 drops of 6 M H2SO4 to the precipitate. The formation of a white precipitate, BaSO4, indicates the presence of Ba2+. C-1 test Confirmation of Calcium ions (Ca2+) Before this analysis is performed, the B-1 test should be performed. The solution set aside for additional Group IV ion tests (in the test tube labeled C-1) will be used in this analysis. To the solution, add ~15 drops of 6 M NH3 until the solution is basic (analyze with pH paper). If a precipitate forms, centrifuge the mixture, then carefully pipet the supernatant into a clean test tube. Add 10 drops of 1 M potassium oxalate (K2C2O4) and mix. Warm the test tube briefly in a hot water bath then let cool. The formation of a white precipitate, CaC2O4, at any stage after the addition of the reagent is confirmation of the presence of Ca2+. 6 I-1 test Confirmation of Iron ions Before this analysis is performed, the N-1 test should be completed. The solution containing the remaining Group III ions (set aside and labeled I-1 from the N-1 test) will be used in this analysis. To the solution containing the remaining Group III ions, add 2 drops of 0.2 M potassium thiocyanate (KSCN) to the solution. The solution will turn a dark blood red if iron ions are present. L-1 test Confirmation of Lead (II) ions (Pb2+) Lead chloride is almost three times more soluble in hot water than cold. One may use this as a basis for separating it from the other Group I ions. To the precipitate from the Group I ions separation, add 15 drops of deionized water. Place the test tube in a hot water bath. Stir with a stirring rod and heat for 1 minute. Centrifuge and pipet the hot liquid into a test tube. Repeat this procedure two more times and combine the supernatant. Save the precipitate for additional Group I analyses (label the test tube appropriately). To the combined supernatants, add 3 drops of 1 M K2CrO4. A yellow precipitate (PbCrO4) confirms the presence of Pb2+. N-1 test Confirmation of Nickel ions (Ni2+) To the precipitate from the Group III ions, add 10 drops of 6 M HCl and heat the mixture for ~1 min to dissolve the solids. Cool the solution to room temperature. Separate half of the solution into a test tube labeled I-1 test and set aside. Carefully add 6 M NH3 until the solution turns basic (a few drops). This produces the Ni(NH3)62+ complex ion. Add 5 drops of deionized H2O followed by 4 drops of 1% dimethylglyoxime to the solution. Ni2+ is confirmed by the formation of a scarlet to strawberry-red precipitate. If the precipitate does not form immediately, stir occasionally for ~5 minutes. S-1 test Confirmation of Silver ions (Ag+) Silver forms a soluble complex ion with aqueous ammonia. The presence of silver is confirmed by dissolving the precipitate from the Group I ions separation in 6 M NH3 then re-precipitating the chloride using acid. To the precipitate from the Group I ions separation, first perform the L-1 test. From the precipitate from the L-1 test, add ~20 drops of 6 M NH3 to dissolve the solid. Add ~12 drops of 6 M HNO3 until the solution is acidic (use litmus paper to test the acidity, if needed). A white precipitate (AgCl) confirms the presence of Ag+. For the Lab Report Show all of the reactions for both the positive tests and the unknown samples for the G-1, G-3, G-4, B-1, C-1, L-1, and S-1 tests. Discuss the results for all of the tests. Identify the cations in the unknown. 7