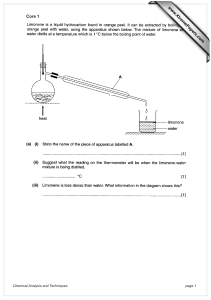
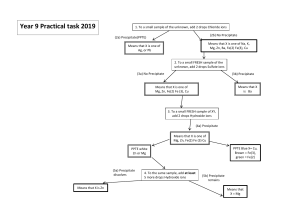
Island School Chemistry A&A 9c Preparation & Analysis Questions ANSWERS 1. Identify each of the following substances by identifying both the positive and negative ions present. The first one has been done for you. CATION (+ ION) test A Yellow flame test B Sodium ions ( Na+) Lilac flame test ANION (- ION) test Name & formula White precipitate with silver nitrate Chloride ions (Cl -) White precipitate with barium chloride Sodium chloride (NaCl) Potassium sulfate K2SO4 Copper(II) carbonate CuCO3 C Blue precipitate with sodium hydroxide Bubbles (of CO2) with acid D Red flame test Yellow precipitate with silver nitrate Lithium iodide LiI E Green precipitate with sodium hydroxide Yellow precipitate with silver nitrate Iron(II) iodide Fe I2 F Brown precipitate with sodium hydroxide White precipitate with barium chloride Iron(III) sulfate Fe2(SO4)3 G Brick red flame test White precipitate with silver nitrate Calcium chloride CaCl2 H Lilac flame test Cream precipitate with silver nitrate Potassium bromide KBr I Blue precipitate with sodium hydroxide solution White precipitate with barium chloride Copper(II) sulfate CuSO4 J Produces an alkaline gas when heated with sodium hydroxide Bubbles with acid Ammonium carbonate (NH4)2CO3 Island School Chemistry A&A 9c 2. Give the tests that you would use to identify the positive and the negative ions in each of the following substances. Also give the result of the test. Formula KCl Test for cation Flame test Result lilac CaCO3 Flame test Orange/red HCl (or any acid) Fizz CuSO4 NaOH Blue ppt Acidified BaCl2 white ppt FeBr2 NaOH Green ppt Acidified AgNO3 cream ppt Flame test Bright red Acidified AgNO3 yellow ppt NaOH, warm gently and UI paper UI blue None!!! Li I NH4NO3 Test for anion Acidified AgNO3 X 3. Identify the substances below: What do I do? I ‘pop’ with a lighted splint Result white ppt What am I? H2 I turn limewater milky CO2 I smell awful and turn damp red litmus blue NH3 I bleach damp indicator paper Cl2 I turn anhydrous copper(II)sulphate from white to blue I relight a glowing splint H2O O2