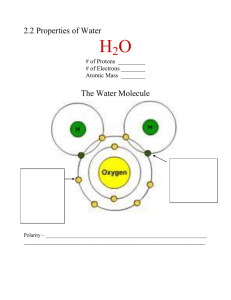
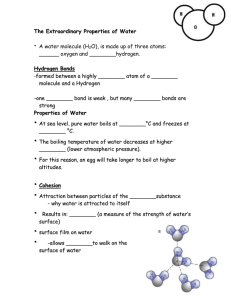
Note Guide If you easily get overwhelmed when reading about something new, I recommend starting with the Chapter Review at the end of every chapter. The reviews are not complete, nor do they contain enough detail to replace a full reading of the chapter, but they will give you an idea of what you should focus on. Take your notes on lined paper. Outline, Cornell-style, or other, it is important that whatever format you use, your notes are interactive. For example, as you do your first read-through, you may want to write questions or annotations on sticky-notes, then transfer those to your paper and address them in your notes. The answers to the Concept Checks are in the Appendix of the textbook. Copying the answers is not very useful for learning and it’s against our academic honesty policy. Paraphrasing the answers in your own words is more effective, and honest. Coming up with your own answers is even better. Writing your own answer, checking against the answers in the appendix, and then annotating your original answer will have the most impact on your learning.🙌 Leave yourself at least a half a page of blank space and label it REVIEW NOTES. We will go over the reading quizzes, and you’ll want to take notes here during the review sessions. Don’t forget you are responsible for adding at least 5 new entries to your glossary per assigned chapter. Surely there will be more than 5 science vocabulary words that you will need to learn, so it is recommended that you define as many terms as you need to. If you would rather make flashcards instead of keeping a glossary, you must keep the cards organized in a folder within your notebook that is kept in the same place as your glossary would be. Electronic flashcards are not accepted. Now onto the note guide for Chapter 3: The unique structure of water is why water is a polar compound. Water’s polarity allows it to engage in hydrogen bonding. The ability to easily form hydrogen bonds is what gives water many unique properties, and life on Earth takes advantage of those properties which is why water is such a biologically important compound. Furthermore, the chemistry of life occurs in a very narrow pH range, so it is important that organisms can maintain a neutral environment. Understanding figure 3.2 is very important. You should be able to draw a water molecule interacting with other water molecules as directed in the “Draw It” box near the figure. Be able to identify and label the partial charges (δ+ and δ-), covalent bonds, and hydrogen bonds. Do Concept Check questions 1-3. The quiz date is: The four properties of water and their respective examples are important. For each of the properties of water, make note on how polarity and hydrogen bonding creates the property. Then take note on why each property is important to life. Do Concept Check questions 1-4. You must understand what a hydrogen ion is and what it represents. You should be able to compare the behavior of strong bases, weak bases, strong acids, and weak acids. The section on pH scale goes into great detail, but the most relevant information to this class is on page 52. Focus on what acids and bases actually do. It’s more important to know how to read the pH scale and understand what the numbers on the pH scale indicate than it is to be able to calculate hydrogen ion concentration. You must be able to describe how buffers moderate pH. Blood pH and ocean acidification are common settings that may be given to you on tests for you to demonstrate your understanding of buffers and pH. Make sure you are able to track which molecules are taking up or releasing hydrogen ions in the textbook examples, and how that either changes the pH, or helps to maintain the pH. Do Concept Check question 3 only. Tip: Compare the acetic acid in the question to the carbonic acid in the blood and ocean examples. The acetic acid should behave similarly, so take a guess at how to answer this question. In this class you will not be asked to calculate molecular mass, moles, and molarity. You do need to remember that molarity is the unit of concentration of an aqueous solution. It’s also helpful to review the difference between solute and solvent for this unit and the next unit. The section about life on other planets is interesting, but it’s unlikely you’ll be asked about this section on tests. Although, it is possible that the AP exam may ask you hypothetically what conditions may indicate the presence of life on other planets. You will not be asked to calculate pH.