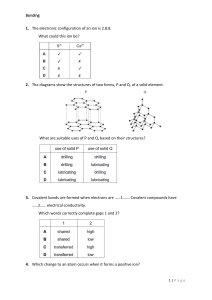
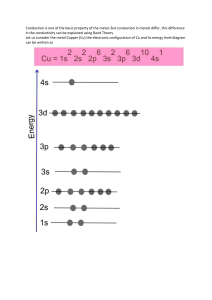
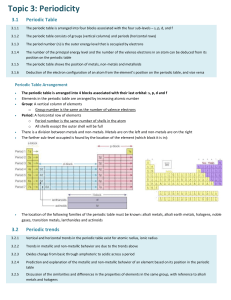
CHEMISTRY The metallic bonding is non-localized, metals tend to be malleable (can be beaten into a sheet), and ductile (be drawn into a wire), and (generally) electrically conductive. That is the metallic bond can be maintained by the electron glue that binds the positively charged metal atoms together, even though they, the positive ions, can change their position with respect to each each other • • • • In metals, valence electrons are free to move between atoms, forming a ‘sea’ of delocalised electrons. These electrons surround a lattice of positive metal ions. These lattices are held together by metallic bonds, which are strong forces of attraction between positively charged metal ions and negatively charged valence electrons. Since they have a similar arrangement of atoms all metals properties include lustre, electrical conductivity, thermal conductivity, malleability and ductility.