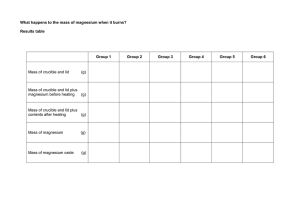
Magnesium Oxide – Percent Yield Lab Aushkanwar Singh Toor SCH 3U Harjot Singh Sandhu Wednesday, February 6th, 2019 Abstract The purpose of this report was to prepare a sample of magnesium oxide and calculate its percent composition compare this to the theoretical percent composition. This was done by heating a crucible with 2 strips of magnesium in it. Then water was added to the product, and it was then reheated. All measurements were recorded in the observations. The magnesium ribbons reacted vigorously with the heat. When it was exposed to oxygen, then the magnesium would ignite even more. The end result was a product that was solid and flaky and light grey in colour. After this, calculations were made to figure out the percent yield, theoretical yield and percent composition. It was discovered that the percent yield was less than 100%, meaning that all of the limiting reactant was not used, thus proving the hypothesis correct. Introduction Percentage yield is a significant stoichiometry concept needed to understand the results from this experiment. There are two concepts related to percentage yield which are, theoretical yield and actual yield. When all of the limiting regent is converted into the anticipated product, then the maximum possible yield of the product is identified; this is termed as theoretical yield. Theoretical yield is a prediction based on the stoichiometry of a reaction which states how much of the product should be formed. It is accomplished if 100% of the limiting regent is converted into products. However, in reality, theoretical yields are hardly achieved, and instead the actual yield, the amount which is the amount of the product physically obtained through an experiment or industrial process, is generally less. The meaning of this is that some or most of the limiting reactant did not become part of the collected product. (DiGiuseppe, M.,2011) The calculation for percentage yield is: A reason for the actual yield being less than the theoretical yield could be the very nature of the reaction. For example, ammonia, which is an important industrial chemical, spontaneously decomposes as it forms. This is called a reverse reaction and it ends up limiting the actual yield of ammonia. Even though chemists have determined the temperature, pressure, and catalyst that give the best yield of ammonia, the actual yield is never equal to the theoretical yield. (DiGiuseppe, M.,2011) Nitrogen and hydrogen react to form ammonia. As the reaction continues, ammonia decomposes. Another important concept needed to understand the results from the lab is percent composition. The ratio of atoms or ions in a sample of a compound can be determined if the relative masses of its elements are known. Using this information, the molar masses can be used to convert relative masses to numbers of entities. Relative masses are usually given as percentage composition. The percentage of each element in the compound, by mass, is known as percentage composition. Percentage composition is not the same as the ratios of atoms of elements. (DiGiuseppe, M.,2011) The calculation for percentage composition is: Purpose The purpose is to prepare a sample of magnesium oxide and calculate it’s percent composition and compare this to the theoretical percent composition. Hypothesis If a sample of magnesium oxide is prepared, then the actual yield will be lower than the theoretical yield, because of impurities. Materials - Crucible and lid Ring clamp Bunsen burner Retort stand Dropper 2 magnesium ribbon pieces Electronic balance Water Crucible tongs Metal Mesh Procedure 1. The retort stand, ring clamp clay triangle, and Bunsen burner were set up as shown. Have the setup sketched. 2. Two small strips of magnesium were obtained. The strips were lightly sanded if they were not already shiny. 3. The crucible and lid were cleaned and dried. To ensure that they were dried properly, a cool blue Bunsen burner flame was used to dry them off. 4. After the crucible cooled for 5 minutes, the mass of the crucible and lid were measured and recorded. 5. The two magnesium ribbons were loosely coiled and placed at the bottom of the crucible. 6. The mass of the crucible, lid and contents was measured and recorded. 7. With the lid of the crucible fully on, the crucible was slowly heated over a hot Bunsen burner flame. When large quantities of smoke began to escape from the crucible, the flame was moved away. The lid was periodically lifted while the crucible was being heated to ensure that enough oxygen got into the crucible. 8. After the crucible stopped smoking, the lid was removed and the contents of the crucible were heated to redness for 8 minutes. 9. The Bunsen burner was removed and the crucible was let to cool for 3 minutes. 10. 10 drops of water were added. Any unusual odors were wafted and noted down. The observation for this step were recorded. 11. The crucible was heated over a cool blue Bunsen burner for 5 minutes until it was completely dried (splattering was avoided). 12. For 5 minutes the crucible was let to cool down. The mass, contents and the lid of the crucible were measured. The result was recorded. 13. All apparatus was cleaned. Observations Table 1 - The state of magnesium during different times of the experiment Stage of the experiment Before the start of the experiment Condition of the magnesium The magnesium had a dark grey color. It was a little lustrous. It was malleable and ductile. It was solid and hard. While the magnesium ribbons were being heated The magnesium burned very brightly. At some instances it was difficult to examine what was happening to the magnesium, because there was too much light. When the lid was lifted off periodically a lot of smoke would escape. Also, the magnesium would ignite when the lid was lifted off. After the magnesium was done being heated After adding 10 drops of water Once the combustion reaction was completed, the magnesium would not ignite when exposed to oxygen. After the magnesium cooled down, it looked like ash. The magnesium was white and looked very brittle. When the water was added to the magnesium, it created a pungent odour and it made the magnesim ash a sage green colour. It look like a thick paste. After reheating the The product is a solid that is flaky and light grey in colour. All mixture of water and the water seems to have evaporated. magnesium. Table 2 – Masses of crucible and lid at different times of the experiment Mass of empty crucible and lid 52.8 g Mass of lid 28.3 g Mass of crucible 24.5 g Mass of crucible, lid and magnesium ribbons 53.0 g Mass of MgO and crucible after heating with lid 53.5 g The mass of the empty crucible combined with lid was 52.8 grams. The mass of the crucible alone was 28.3 grams. Therefore, the mass of the lid came out to be 28.3 grams. When the two pieces of the magnesium were placed at the bottom of the crucible, the mass, including the lid was 53 grams. The last measurement was of the magnesium oxide, combined with the crucible and lid, which came out to be 53.5 grams. Discussion The hypothesis was that if a sample of magnesium oxide is prepared, then the actual yield will be lower than the theoretical yield, because of impurities. It was proven correct, because the actual yield was lower than the theoretical yield. A factor that could cause the theoretical yield to be more than the actual yield is impurities. Chemicals that are used in investigation come in a wide range of purities, meaning they are infrequently 100 % pure. Therefore, the mass of the reactant in any sample of the initial chemical is a lesser amount than the measured mass. If the impurities are not taken into account, then the amount which was predicted for the theoretical yield will be impossible to obtain. Some chemicals become more impure as they age, especially if they are not stored adequately, like sodium hydroxide. Another example is magnesium; an oxide layer forms on the magnesium if it is exposed to air. The mass of the strip of “magnesium” will increase cause of this. (DiGiuseppe, M.,2011) 1. With reference to the procedure, discuss any sources of error. Note: Errors are not mistakes. Correct your mistakes - better yet, avoid making them. Errors are aspects of the procedure that can alter the outcome, but cannot be avoided due to the nature of the equipment used or the requirements of the reaction. The only source of error related to the procedure is the exposing of magnesium to oxygen. This increased the mass of the magnesium, making the theoretical yield impossible to reach. A suggestion for this could be to limit the exposure of magnesium by only using it right before beginning the experiment. All other errors were caused by humans and could avoided through carefulness. 2. Why is the percent yield and percent composition important in industry? What applications are there to industry? Percent yield is important, because chemists require a measurement that specifies the effectiveness of a reaction and percentage yield gives that information. Percentage yield is important in the manufacturing of products. A lot of time and money is expended to improve the percent yield for chemical production. When intricate chemicals are produced by numerous different reactions, one step with a low percentage yield can cause a large waste of reactants and needless expense. (CK-12 Foundation, 2018) The reaction conditions which are used are chosen to acquire an acceptable yield of product in an adequate time. For example, if it took several weeks or months to achieve a very high yield, then there would be very little profit. (GCSE Chemistry (Single Science) Making fertiliser - OCR Gateway - Revision 5., 2018) If the temperature is increased, the equilibrium position, a measure of the relative concentrations of substances in an equilibrium, showing if the products of reactants are more in quantity, moves in the direction of the endothermic reaction. This means that it moves to the left in the Haber process. The Haber Process is an industrial chemical process that makes ammonia by reacting nitrogen and hydrogen together. A low temperature produces a low rate of reaction, so a temperature of 450 °C is chosen. This temperature is low enough to achieve an acceptable yield and high enough to complete in an acceptable time. The equilibrium position moves in the direction of the fewest molecules of gas if the pressure is increased. That means it moves to the right in the Haber process. Still, it is very expensive to achieve high pressures, because stronger equipment is required, and more energy to compress the gases. Therefore, a pressure of 200 atmospheres is chosen. This is high enough to attain an adequate yield and low enough to keep the expenditures low. (GCSE Chemistry (Single Science) - Making fertiliser - OCR Gateway - Revision 5., 2018) Graph 1 - The yield of ammonia changes with changes in pressure and temperature Catalysts do not change the equilibrium concentrations of reacting substances in reversible reactions are not change by catalysts, but the time taken to reach the equilibrium is reduced. Iron is a cheap catalyst used in the Haber process. It helps to achieve an acceptable yield in an acceptable time. (GCSE Chemistry (Single Science) - Making fertiliser - OCR Gateway Revision 5., 2018) Percent composition is used in many applications relating to industry, pharmacy, and construction. In jewelry, percent composition is used to decide the precise percentage of a specific metal in an alloy. Alloys are mixtures of different metals blended together to form useful products. Dissimilar to compounds, the percentage composition of an alloy can fluctuate. For example, stainless steel which used to make cutlery, contains approximately 10 % chromium by mass. Surgical stainless steel is made up of at least 18 % chromium. By augmenting the proportion of chromium, steel is made more resistant to corrosion, an important property for knee replacements. The percentage composition percentage of substances in mixtures can also be considered. The food industry most of the times is related to the water, salt, or fat proportions in their products. (DiGiuseppe, M.,2011) Percent composition is also used to discover the right number of specific elements in a mixture of concrete, and to determine the correct amount of an element or mineral in specific pharmaceuticals. (Percent Composition By Mass, 2018) 3. Calculate an average experimental percent composition based on the results from all groups. Group One: %Mg in MgO = 100% * (Mass of Mg) / (mass of MgO) = 100% * (0.2g) / (0.7g) = 28.57% %O in MgO = 71.43% Group Two: %Mg = (0.2g/0.3g) *100% = 66.67% %O = (0.1/0.3) *100% = 33.33% Class average for Oxygen = 53.28% Class average for Magnesium = 47.62% 4. Consider the following set of reactions in your discussion of the procedure. Why does (b) occur and what is the purpose of (c) and (d)? a) 2Mg + O2 —> 2MgO b) 3Mg + N2 —> Mg3N2 c) Mg3N2 + 6H2O —> 2NH3 + 3Mg(OH)2 d) Mg(OH)2 —(with heat) —> MgO + H2O Reaction b) occurs because the air is made up of 78% nitrogen, so the nitrogen in the air and the magnesium react together to make magnesium nitride. The purpose of reaction c) is to create ammonia and magnesium hydroxide which neutralizes stomach acid for those with acidity. The purpose for reaction d) is that when magnesium hydroxide is heated, it decomposes into magnesium oxide and water, which is what the purpose of this lab is. Conclusions The hypothesis stated that if a sample of magnesium oxide is prepared, then the actual yield will be lower than the theoretical yield, because of impurities. This was true, because the actual yield was 0.3 grams, while the theoretical yield was 0.324 grams. This happened due to impurities, especially when the magnesium was exposed to air, because the mass of the magnesium increases cause of this. The law of definite proportions states that a compound always contains the same proportion of elements by mass. The class average for magnesium was 47.64% while for oxygen it was 53.28%. The law is proved correct, because the difference between these two number is not that great when converted into grams. Questions 1. Mass of magnesium ribbons = (Mass of crucible, lid and magnesium ribbons) – (Mass of empty crucible and lid) = 53.0g – 52.8g = 0.2 g There were two magnesium ribbons so the mass of one will be 0.1 grams. Mass of MgO = (Mass of MgO and crucible after heating with lid) – (Mass of crucible, lid and magnesium ribbons) = 53.1g – 52.8g = 0.3g The mass of magnesium oxide is 0.7g. 2. 2Mg + O2 2MgO 0.2g * 1mol / 24.035 g/mol = 0.008 mol 0.008mol * 2mol/2mol = 0.008mol 0.008mol * 40.6088g/mol = 0.324g Percent Yield= (actual yield/theoretical yield) x 100% Percent Yield = 0.3/0.324 *100% = 92.59% 3. %Mg = (0.2g/0.3g) *100% = 66.67% %O = (0.1/0.3) *100% = 33.33% 4. 100% * (subscript * average atomic mass) / (subscript * average atomic mass) 100% (2*24.305) / (2*40.3044) = 100% (48.61) / (80.6088) = 100% * 0.603 = 60.3% 5. Experimental value = g Theoretical value = 0.324g %discrepancy = (Experimental value - theoretical value / theoretical value) * 100% %discrepancy = (0.3– 0.324/0.324) *100% = 7.41% References CK-12 Foundation. (n.d.). Retrieved from https://www.ck12.org/book/CK-12-ChemistryConcepts-Intermediate/section/12.9/ DiGiuseppe, M. (2011). Nelson chemistry 11: University preparation. Toronto, Ont.: Thomson/Nelson. GCSE Chemistry (Single Science) - Making fertiliser - OCR Gateway - Revision 5. (n.d.). Retrieved from https://www.bbc.com/bitesize/guides/zxy9ng8/revision/5 Percent Composition By Mass - Lesson. (n.d.). Retrieved from https://www.helpteaching.com/lessons/78/percent-composition-by-mass