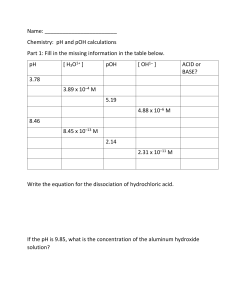
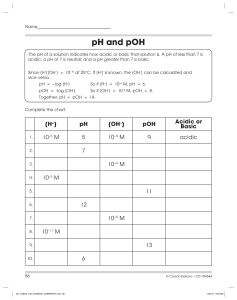
pH Scale & Calculations Worksheet
advertisement

Name: __________________________________ Date: _______________ Period: ____ WS pH Scale & Calculations 1. A water molecule that loses a hydrogen-ion (H+) becomes a __________________ ion with the formula _______. 2. A water molecule that gains a hydrogen-ion (H+) becomes a __________________ ion with the formula _______. 3. The reaction represented below in which water molecules produce ions is called the _________________________ of water. 4. A(n) __________________ solution is one with more [H+] than [OH–]. A(n) __________________ solution is one with more [OH–] than [H+]. 5. 6. Match the type of solution with its hydrogen-ion concentration: ____ acidic A. less than 1.0 x 10–7 M [H+] ____ neutral B. greater than 1.0 x 10–7 M [H+] ____ basic C. equal to 1.0 x 10–7 M [H+] pH is a measure of the concentration of ________________________________ in a solution. Mathematically, pH is the negative ____ of __________________ concentration. OR pH = 1 7. 8. Match the type of solution with its pH: ____ acidic A. pH > 7 ____ neutral B. pH = 7 ____ basic C. pH < 7 Write the following substances on the appropriate line along the pH scale that estimates the pH: ammonia 9. vinegar blood stomach juices soap DI water WITHOUT a CALCULATOR, write the pH or hydrogen-ion concentration of these solutions: [H+] in M pH acidic, basic, or neutral A. 1.00 x 10–7 M ____ _________ B. 1.00 x 10–1 M ____ _________ C. _________ M 9 _________ D. _________ M 3 _________ E. 3.20 x 10–10 M ≈ ____ _________ (approximately) For #10-11: SHOW ALL WORK and use a Calculator 10. Calculate the pH for a solution of 2.40 x 10–10 M [H+]. 11. Calculate the pH for a solution of 8.30 x 10–5 M [H+]. 2 12. pOH is a measure of the concentration of ________________ in a solution. Mathematically, pOH is the negative ____ of __________________ concentration. OR pOH = 13. What is the pOH of a neutral solution? _____ 14. Calculate the pH for a solution of pOH = 6. Calculate the pOH for a solution of pH = 11.5 15. Complete the table below. (You may show your work on the back, or show no work) pH A. 3.80 B. 2.25 pOH C. 1.50 D. 8.14 [H+] E. 1.26 x 10–4 M F. 2.04 x 10–4 M [OH–] G. 4.57 x 10–8 M H. 3.16 x 10–3 M acid or base 3