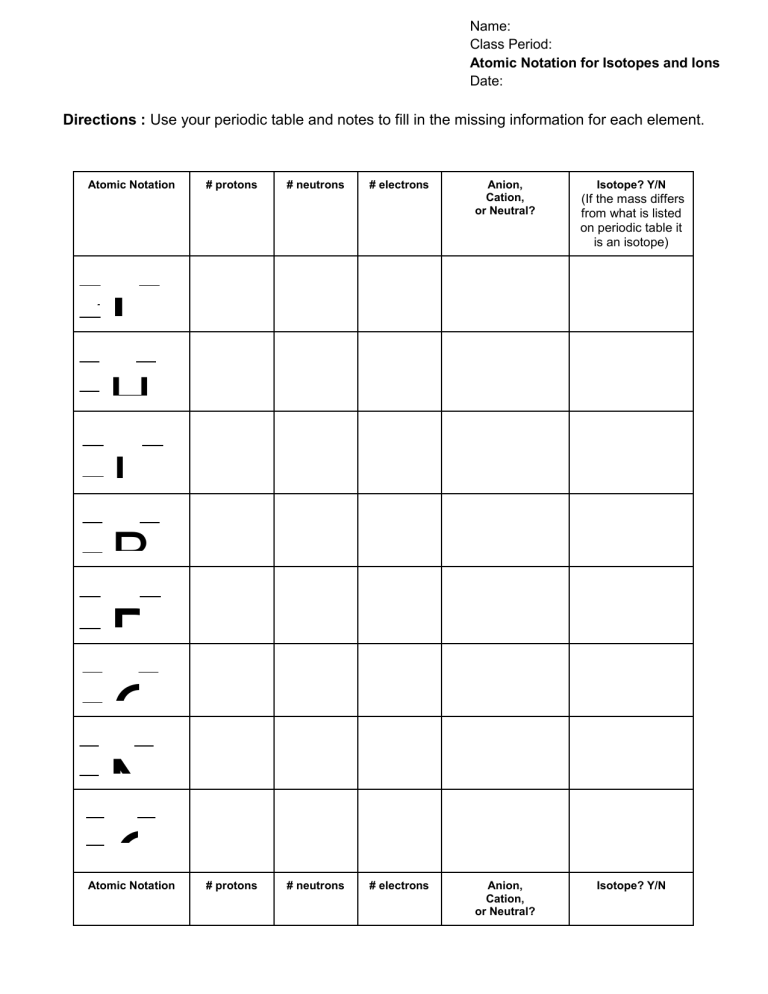
Name: Class Period: Atomic Notation for Isotopes and Ions Date: Directions : Use your periodic table and notes to fill in the missing information for each element. Atomic Notation 4 1 # protons # neutrons # electrons Anion, Cation, or Neutral? Isotope? Y/N (If the mass differs from what is listed on periodic table it is an isotope) - H H 7 + 3L 9 + 4B + B 6 C N O 5 1 - 1 1 Atomic Notation # protons # neutrons # electrons Anion, Cation, or Neutral? Isotope? Y/N 1 9 2 - F N 2 + 1N 2 + M A 3 S 3 3 P S- C 4 A