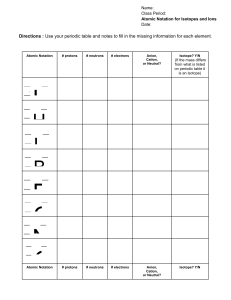
A. # of protons A. # of protons
advertisement

A. B. C. D. E. # of protons Atomic Number Mass Number Symbol of element Isotope notation Energy Level A. # of protons B. Atomic Number C. Mass Number D. Symbol of element E. Isotope notation Sublevels Electrons per Sublevel A. # of protons B. Atomic Number C. Mass Number D. Symbol of element E. Isotope notation Total Electrons 1 2 3 4 Write the electron configuration and orbital notation for the following isotopes: 1. 2. 3. 4. 5. 6. 7. 8. He-4 Li-7 N-14 F-19 Ne-20 Ca-40 Br-70 Mg-24 1. Please create a change of phase diagram for the substance Phosphorus. Phosphorus is an element with a boiling point of 280 ºC and a melting point of 44ºC. 2. List as many physical properties of water that you can think of. Do the same for bleach. Which traits do they share? Where do they differ? 3. The density of Aluminum is 2.7g/cm3. If you have 500g of aluminum, what volume of space does it occupy? 4. Determine the density of a substance that has a mass of 45.9g and occupies 5.3cm3. For the following pairs of elements: 1. Classify them as metals or nonmetals. 2. Decide if they are stable or unstable. 3. If they are unstable determine the type of bond that would form between then. NOTE: You may need more than one atom of a particular element to complete the molecule! 4. If the bond is ionic: draw the Lewis Dot structures and indicate where the electron transfer occurs. Finally write the resulting charge of each ion formed. 5. If the bond is covalent: draw the Lewis Dot structures, circle the potential shared pairs of electrons, rewrite the Lewis Dot structures with the shared pairs in between the symbols for the atoms, and finally represent the shared pairs with solid lines. Identify the type(s) of covalent (single, double, triple) existing. 1. Na F 2. Mg O 3. He Ne 4. O O 5. O H 6. C Cl 7. N N 8. Li Cl 9. Fr Br 10. C O Please solve the following Gas Law problems using the Combined Gas Law. Follow our format: Write the formula, create a list of what is given (remember to convert Temperature to Kelvin), eliminate what you don’t need, substitute into the formula and solve (include units)! 1. 4L of Helium gas at room temperature is heated to 100ºC. What is the new volume of the gas? 2. Under normal conditions 10L of air is under 101.3kPa of pressure. If the pressure is increased to 202.6kPa what is the new volume of the air? 3. Please write the formulas for Boyle’s Law and Charles’ Law. Also include what these laws state. 4. A gas at 0ºC has a volume of 2.5L. The gas is heated to 50ºC. How much space does the gas occupy now? 5. A new volume of O2 gas was measured to be 12L after it was heated from 25ºC to 75ºC. What was the original volume of the O2 gas? 6. Normal air pressure on 6L of N2 gas is 1atm. If the gas is condensed into a volume of 1L, what is the new pressure of the gas? 7. The pressure on 4L of air is increased from 101.3kPa to 405.2kPa. How much space would the gas take up under this pressure?