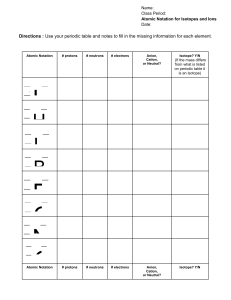
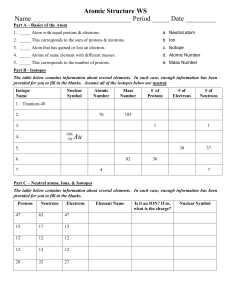
Atoms and Elements Self-Assessment Name ____________________________________________________________ 1. Two samples of a compound containing element A and B are decomposed. The first sample produces 15 g of A and 35 g of B. The second sample produces 25 g of A and what mass of B? 2. A compound containing only carbon and hydrogen has a carbon-to-hydrogen mass ratio of 11.89. Which carbon-to-hydrogen mass ratio is possible for another compound composed only of carbon and hydrogen? 3. Determine the number of protons and neutrons in the isotope Fe-58. 4. An isotope of an element contains 82 protons and 122 neutrons. What is the symbol for the isotope? 5. Determine the number of electrons in the Cr3+ ion. 6. Which pair of elements do you expect to be most similar in their chemical properties? a. K and Fe b. O and Si c. Ne and N d. Br and I 7. Which element is a transition metal? a. Se b. Mo c. Sr d. Ba 8. What is the charge on the ion most commonly formed by S? a. 2+ b. + c. d. 29. A naturally occurring sample of an element contains only two isotopes. The first isotope has a mass of 68.9255 amu and a natural abundance of 60.11%. The second isotope has a mass of 70.9247 amu. Find the atomic mass of the element. 10. Label the following. 11. Label the following. 12. Fill in the table with the missing information: Isotope Isotope Name Symbol Iodine-129 Atomic Number Atomic Mass 19 41 # of protons # of electrons # of neutrons Radium228 13. If 32 g of carbon and 16 g of hydrogen goes into a reaction, how many grams of carbon will come out? 14. Which statement about sub-atomic particles are false? a. If an atom has an equal number of protons and electrons, it will be chargeneutral. b. Electrons are attracted to protons. c. Electrons are much lighter than neutrons. d. Protons have twice the mass of neutrons. 15. Which statement about sub-atomic particles are false? a. Protons and electrons have charges of the same magnitude but opposite signs. b. Protons have about the same mass as neutrons. c. Some atoms don’t have any protons. d. Some atoms don’t have any neutrons. 16. Determine the number of protons and electrons in each ion. a. Ni2+ = b. S2- = c. Br- = d. Cr3+ = 17. Predict the charge of the ion formed by each element. a. O b. K c. Al d. Rb 18. Classify each element as an alkali metal, alkaline earth metal, halogen, or noble gas. a. F b. Sr c. K d. Ne e. At 19. An element has two naturally occurring isotopes. Isotope 1 has a mass of 120.9038 amu and a relative abundance of 57.4%, and isotope 2 has a mass of 122.9042 amu. Find the atomic mass of this element and identify it. 20. An element has four naturally occurring isotopes with the masses and natural abundances given in the following table. Find the atomic mass of the element and identify it. Isotope Mass (amu) Abundance (%) 1 135.90714 0.19 2 137.90599 0.25 3 139.90543 88.43 4 141.90924 11.13