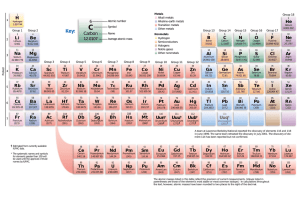
periodic table

The Periodic Table
December 16, 2014
DO NOW AND OBJECTIVE:
• Do Now: What number on the periodic table tells the number of electrons?
Neutrons? Atomic Weight?
• Objective: SWBAT Explain how chemists began to organize the known elements
Organizing the table
• Some of this should be a review!!
• Early chemists used the properties of an element to sort them into categories
Dobereiner
• In 1829, J.W. Dobereiner used the properties to classify the elements into triads.
• Dobereiner discovered that the relative atomic mass of the middle element in each triad was close to the average of the relative atomic masses of the other two elements.
• This gave other scientists a clue that relative atomic masses were important when arranging the elements
Dobereiner’s Table
John Newlands
• 1864 ; Great Britain
• Developed the “Law of Octaves” which showed that every eighth element shared a resemblance.
• Problems:
• not all elements are in the correct column
• He didn’t leave room for new elements!!!
• Not readily accepted, but is now recognized as important in modern chemistry
Newlands Table
Mendeleev’s Table
• Based his table on a set of repeating properties
• He arranged the elements by increasing atomic mass by writing characteristics on to note cards and then continuing to rearrange them
• Do we still do this??? Look at your table
William Ramsey
• 1894 Great Britain
• Found the Nobel Gases
• Remember that the nobels are inert…that is…they do not like to mix with the commoners (the other elements)
Henry Moseley
• 1914; Great Britain
• The Periodic Law states: When elements are arranged in increasing atomic number, there is a repetition of their physical and chemical properties.
Current Periodic Table
• Elements are placed in increasing atomic number, which means that they are shown in order of increasing number of protons (and electrons)
• Those that share properties are in the same column of the table ( columns are up and down)
DO NOW AND OBJECTIVE:
• Do Now: How did Mendeleev establish his table, and is it still validated?
• Objective: SWBAT explain how the elements are arranged on the table due to their characterisitcs
Add to your table
• Alkaline Earth Metals
• Alkali Metals
• Transition metals
• Metalloids
• INCLUDE ASTATINE
• Non-metals
• Halogens (family group 7)
• Nobel Gases
Metals and Nonmetals
December 17, 2014
What is metal vs nonmetal?
METAL
Three broad classes of elements
• Metals: are generally
• Good conductors of heat and electricity
• Solids at room temperature (except which?)
• Have a sheen when polished
• Ductile (can be drawn thin into wires)
• Malleable (hammered into sheets)
• Nonmetals: are generally
• In the upper right corner except for hydrogen
• Gases at room temperature
• Few are solids (sulfur and phosphorus)
• One is liquid (bromine)
• Poor conductors of heat and electricity
• Brittle so they shatter when hit
• Metalloids: generally “in the middle”
• under some conditions they act like metals and under others they act like nonmetals
• They sit around the staircase
Add to your table
• Alkaline Earth Metals
• Alkali Metals
• Transition metals
• Metalloids (semiconductors in your book)
• INCLUDE ASTATINE
• Non-metals
• Halogens
• Nobel Gases
Classifying Elements
December 18, 2014
DO NOW AND OBJECTIVE:
• DO NOW: Briefly explain the three categories of elements
• OBJECTIVE: SWBAT Describe the information in a periodic table.
There are subunits of metals, metalloids and nonmetals
• Elements in Group 1A are called alkali metals
• Elements in Group 2A are called alkaline earth metals
• Elements in Group7A are called halogens
Representative elements
• Are in groups 1A through 7A
Transition Elements
• Are in the main body of the table
• They are the only ones with electrons in the d sublevel
TRENDS!
• These should be a review for you!
• Atomic size
• An atomic radius is one half of the distance between the nuclei of two atoms of the same elements when they are joined.
• In general, the atomic size increases as you move down the group (more electrons = more shells=bigger size “shielding”)
• It also decreases as you move left to right across the table (protons have strong pull on the electrons, and pull them close to the nucleus)
• Ionization energy
• It is the energy needed to rip an electron off of an atom (opposite of electronegativity)
• It decreases as you move from top to bottom
(more shells= greater distance from nucleus=less pull )
• It increases as you move left to right (think back to the positive pull of more protons on the outer electrons)
• Electronegativity
• Is the ability of an atom to pull electrons to itself from somewhere else (opposite of ionization energy)
• It decreases from top to bottom (many shells = further from the nucleus = easier to lose than to gain electrons)
• It increases left to right (shells are more filled = they really want to get that last one to fill the shell = will steal from anybody)
• Ionic size
• Increases from top to bottom (more electrons
= more shells = bigger size)
• BUT, two trends are seen as you move left to right
• As you move left to right through the transition metals, you will decrease in size
• As you move through metalloids and nonmetals, the size increases
Using what you learned
December 19-22, 2014
DO NOW AND OBJECTIVE:
• DO NOW: Explain the difference between electronegativity and ionization energy
• OBJECTIVE: SWBAT demonstrate their knowledge of the material covered in this unit by completing worksheets that reflect the information
• Quiz on Tuesday!