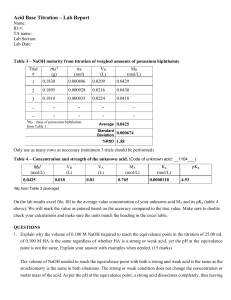
CHEMISTRY FOURTH LABORATORY REPORT ACID-BASE TITRATION Written by: Amelia Quinta Jasmine (CHE) Hanifa Karmelia (FT) Syadza Luthfiyya (FT) Date of Experiment: March 18th, 2016 Date of Submission: April 1st, 2016 Department of Chemical Engineering Department of Food Technology Faculty of Life Science International University of Liaison Indonesia 1.1 Purpose In this experiment, the titration of 2 solutions such as CH3COOH and NaOH was performed to determine the concentration of an acid solution. 1.2 Abstract Basic acid-base titration is generally used to obtain the molarity of a solution given the molarity of other solution that involves neutralization between acid and base. This experiment was done to determine the concentration of the acid solutions. Identifying concentration of an acid solution was given attention in the experiment. The whole experiment dealt with the acid and base solutions. Throughout the experiment, the concentration of 5 ml of NaOH was dissolved with deionized water. The solution was moved inside the burette. Then 5 ml of CH3COOH was dissolved with deionized water inside the beaker glass, then 5 drops of Phenolphtalin was dropped gently using pipette. It was mixed using the stirrer and placed below the burette. Then NaOH was poured into the beaker glass. The solution started to change color. Finally, after the experiment, it was known that the average of the second try and final try solution was 45.40 mL. 1.3 Tools and Materials • • • • • • • • • • Beaker glass Burette Pipette Erlenmeyer flask Magnetic stirrer Stirrer 0.05 mL of NaOH 5 mL of CH3COOH (vinegar) Deionized water 5 drops of Phenoftalein 1.4 Procedure Before the experiment was started, the neutralization reaction of CH3COOH + NaOH had to be known first. The molarity of NaOH was given by the instructor (0.05 M). It can be done by using this calculation: 1 CH3COOH + 1NaOH 1CH3COOH + 1H2O [ Z= mol/l] 5 ml [0.05 M] vol = 45.40 mL Then, n of NaOH and Z of CH3COOH had to be determined. It can be done by using this calculation: n NaOH =Mxv = 0.05 M x 45.40 mL = 2.27 mol 2.27 𝑚𝑜𝑙 Z CH3COOH = 5 𝑥 10−3 𝐿 = 454 mol/L 0.454 mol/l = 0.454 𝑚𝑜𝑙 𝐶𝐻3𝐶𝑂𝑂𝐻 100 𝑚𝑙 = 27.254 𝑔𝑟 𝑚𝑙 Then, the Mr and gr of CH3COOH were calculated. We had to look up to the periodic table to see for the mass for each elements. The calculation can be done as follows; Mr CH3COOH = (2 x 12.001) + (4 x 1.00794) + (2 x 15.994) = 60.03256 gr/mol Gr CH3COOH = 60.03256 gr/mol x 0.454 mol = 27.25478224 gr For the experiment, firstly, deionized water was put into burette to clean up the burette twice. It was filled in until a half burette. Then, 0.05 mL of NaOH was put into burette to clean up again the burette. After that, 0.05 mL solution of NaOH was put into the burette for the titration. 5 mL of vinegar then was taken by using pipette and was put into a beaker glass. Deionized water later was added into the beaker glass for about 10 mL. Then 5 drops of Phenoftalein was added by using pipette into beaker glass. The beaker glass was put above the stirrer, and the magnetic stirrer was used to mix the solution. It was done for about 350 rpm. Then the burette was put above the beaker glass. After it was stirred for a minute, NaOH was started to drop slowly inside the burette. Then it took for a while until the color of vinegar solution plus Phenoftalein changed into soft pink. 1.5 Results and Discussion 2.1 Results A. First try Start point : 0.30 End mark : 42.80 Volume : 42.50 ml (failed) B. Second try Start mark : 1.20 End mark : 47.50 Volume : 46.30 ml C. Final try Start mark : 1.20 End mark : 45.70 Volume : 44.50 ml Average = 44.50 𝑚𝑙 + 46.30 𝑚𝑙 2 = 45.40 mL 2.2 Analysis of Data The concentration of vinegar was calculated for the experiment back then. It was more like a prediction. It can be done by using this equation: M1V1 = M2V2 0.5 mol/l . V1 = 0.05 mol/l . 100 mL 0.5 . V1 = 0.05 x 100 mL 5 𝑚𝐿 V1 = 0.5 V1 = 10 mL Description: M= Molarity (mol/l) V= Volume (l, which later then converted to mL) 2.3 Discussion Titration is the way of analysis of the measurement of the amount of solution needed to react with substances contained fixed with another solution. In this experiment, the molarity was determined the molarity of NaOH using titration process between CH3COOH solution of 10 ml with 0.5 M NaOH solution. In this titration experiment, 10 ml of CH3COOH solution was titrated with NaOH to produce the equation as follows; CH3COOH + NaOH NaCH3COO + H2O H2O H+ + OH• First titration CH3COOH 5 ml of 0.3205 M put into a glass flask, 5 drops of Penoftalin was added. 50 mL of NaOH was put into a burette, then Penoftalin was dropped slowly until the indicator changes color, and NaOH was reduced up to 42.80 ml mark and the color was dark pink. The first titration was failed due to the our inaccuracy, so it caused the solution became dark pink colored. • Second titration CH3COOH 5 ml of 0.3205 M put into a glass flask, 5 drops of Penoftalin was added. 50 mL of NaOH was put into a burette, then Penoftalin was dropped slowly until the indicator changes color, and NaOH was reduced up to 47.50 ml mark and the color was dark pink. The second titration was a success because the solution became pale pink colored. • Final titration CH3COOH 5 ml of 0.3205 M put into a glass flask, 5 drops of Penoftalin was added. 50 mL of NaOH was put into a burette, then Penoftalin was dropped slowly until the indicator changes color, and NaOH was reduced up to 45.70 ml mark and the color was dark pink. The final titration was a success as well because the solution became pale pink colored. 1.6 Conclusion A titration is a valid form of finding the concentration of a substance within a solution. In this experiment, calculation of concentration must be calculated precisely. If experimenters made a single mistake, it would impact to the whole experiment. That is why cautiousness on observing the titration is a vital thing on this experiment. 1.7 Reference S. Zumdahl and Susan A. Zumdahl, Chemistry, 9th edition Laboratory module (Nugraha, Tutun) Laboratory journals (Jasmine, Karmelia and Luthfiyya)