Periodic Trends Worksheet: Atomic Radius, Electronegativity
advertisement

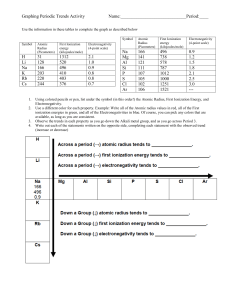
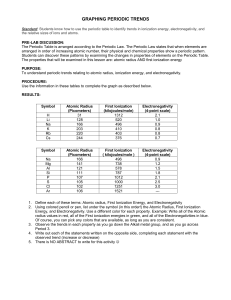
Name: ___________________________________ Date:_____________ Partners’ Name:_______________________________________________________________ Periodic Trends Define the following terms using your book: Atomic Radius: ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ Electronegativity: ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ Ionization Energy: ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ Name: ___________________________________ Date:_____________ Partners’ Name:_______________________________________________________________ Periodic Trends Directions for the activity: In your group, you will need to graph the following data. You are required to have three separate graphs, one for each trend. These graphs should be on graphing paper and you can put more than one graph on one sheet of graphing paper. Element Atomic Number H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Atomic Radius (pm) 37 32 134 125 90 77 75 73 71 69 154 145 130 118 110 102 99 97 Ionization energy (kH/mol 1312 2372 520 899 801 1086 1402 1314 1681 2081 496 738 578 789 1012 1000 1251 1512 Electronegativity 2.1 NA 1.0 1.5 2.0 2.5 3.0 3.5 4.0 NA 0.9 1.3 1.6 1.9 2.2 2.6 3.2 NA Name: ___________________________________ Date:_____________ Partners’ Name:_______________________________________________________________ Periodic Trends Directions: Answer the following questions. Questions: 1. What are trends did you notice? A. Electronegativity: B. Atomic Radius: C. Ionization Energy: 2. Which of the following should have the largest atomic radius according to the trends you noticed? A. Copper or vanadium B. Strontium or antimony C. Arsenic or krypton 3. Which of the following should have the smallest ionization energy according to the trends you noticed? A. Calcium or germanium B. Cesium or bismuth C. Molybdenum or silver 4. Which of the following should have the largest electronegativity according to the trends you noticed? A. Sodium or aluminum B. Rubidium or iodine C. Silicon or chlorine