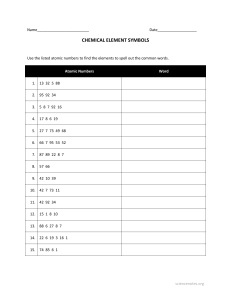
Forest Hills High School Boosang Kim - AP Science Student Name:____________________________ Lab Teacher: _________________________ Day: ___________ Per: _______ Periodic Table Trends - Lab #:____& ____ PAGE 1: Atomic Number vs. Atomic Radius Objectives: Use Table S to determine the atomic radius and electronegativity values for the first 20 elements Graph Atomic Number vs. Atomic Radius Graph Atomic Number vs. Electronegativity Analyze the newly created graphs to determine periodic trends across periods and down groups ● ● ● ● Background Information: The Periodic Table of Elements is a very helpful tool in Chemistry class. We know that elements are arranged in order of increasing atomic number, but the specific location of an element tells us even more information. Elements in the same row, or period, for example, all have the same number of energy levels, whereas elements in the same column, or group/family, have the same number of valence electrons. As you move across a period or down a group, you will notice other trends in atomic radius, electronegativity, and more. Electronegativity is an atom’s affinity, or attraction, for electrons. All elements want a full valence shell, so the closer an element is to filling their valence shell, the higher the electronegativity value. . Graphing Periodic Table Trends: Atomic Radius Checklist In Table 1: Atomic Radius of the First 20 Elements, use Table S to fill in the table below Use the information on the completed Table 1 to create Graph 1: Atomic Number vs. Atomic Radius of the First 20 Elements. (Graph the columns with the *) on PAGE 2 of this lab. RECOMMENDATION: Use pencil for the graph! Label each data point with the element symbol - the first one has been done for you. Use Table 1 and Graph 1 to help you with the atomic radius trend questions (on PAGE 2 of this lab). Table 1: Atomic Radius of the First 20 Elements Atomic Number* Element Name Element Symbol Atomic Radius (pm) * Atomic Number* 1 11 2 12 3 13 4 14 5 15 6 16 7 17 8 18 9 19 10 20 Element Name Element Symbol Atomic Radius (pm)* Forest Hills High School Boosang Kim - AP Science Student Name:____________________________ Lab Teacher: _________________________ Day: ___________ Per: _______ Graph 1: Atomic Number vs. Atomic Radius of the First 20 Elements Question: What do Li, Na, and K have in common, in terms of their location on the periodic table? Question: According to your graph, what happens to the atomic radius as you go from Li to Na to K directly? Question: What do Na, Si, S, and Ar have in common, in terms of their location on the periodic table? Question: According to your graph, what happens to the atomic radius as you go from Na to Ar? Question: Look at the picture of a snowman. What happens to the size of the body as you go from top to bottom? What about the size of the nose when you go from left to right? How does the snowman relate to atomic radius? Forest Hills High School Boosang Kim - AP Science Student Name:____________________________ Lab Teacher: _________________________ Day: ___________ Per: _______ Periodic Table Trends - Lab #:____& ____ PAGE 2: Atomic Number vs. Electronegativity Background Information: The Periodic Table of Elements is a very helpful tool in Chemistry class. We know that elements are arranged in order of increasing atomic number, but the specific location of an element tells us even more information. Elements in the same row, or period, for example, all have the same number of energy levels, whereas elements in the same column, or group/family, have the same number of valence electrons. As you move across a period or down a group, you will notice other trends in atomic radius, electronegativity, and more. Electronegativity is an atom’s affinity, or attraction, for electrons. All elements want a full valence shell, so the closer an element is to filling their valence shell, the higher the electronegativity value. Graphing Periodic Table Trends: Electronegativity Checklist In Table 2: Electronegativity Values of the First 20 Elements, use Table S to fill in the table below Use the information on the completed Table 2 to create Graph 2: Atomic Number vs. Electronegativity of the First 20 Elements. (Graph the columns with the *) RECOMMENDATION: Use pencil for the graph! Label each data point with the element symbol - the first one has been done for you. Use Table 2 and Graph 2 to help you with the electronegativity trend questions . Table 2: Electronegativity of the First 20 Elements Atomic Number* Element Name Element Symbol Electronegativity * Atomic Number* 1 11 2 12 3 13 4 14 5 15 6 16 7 17 8 18 9 19 10 20 Element Name Element Symbol Electronegativity * *NOTE: There are no electronegativity values for Group 18 elements. Periodic Table Trends Summary Chart: Fill in the six blank boxes with either the word INCREASES or DECREASES *COMPLETE THIS CHART AFTER BOTH GRAPHS AND ALL OTHER QUESTIONS ARE COMPLETED* Trend ACROSS PERIOD Trend DOWN GROUP Atomic Radius Electronegativity Ionization Energy Note: You did not graph data for ionization energy. Ionization energy follows the same trend as electronegativity. Values for ionization energy can also be found on Table S. Forest Hills High School Boosang Kim - AP Science Student Name:____________________________ Lab Teacher: _________________________ Day: ___________ Per: _______ Graph 2: Atomic Number vs. Electronegativity of the First 20 Elements Question: What do Be, Mg, and Ca have in common, in terms of their location on the periodic table? Question: According to your graph, what happens to the electronegativity value as you go from Be to Mg to K directly? Question:What do Be, C, O, and F have in common, in terms of their location on the periodic table? Question: According to your graph, what happens to the electronegativity values as you go from Be to F? Question: The front of this lab has a picture of an angler fish. Angler fish attract small fish with the light on their heads, then eat them. How do you think the angler fish relates to electronegativity? Forest Hills High School Boosang Kim - AP Science Student Name:____________________________ Lab Teacher: _________________________ Day: ___________ Per: _______