Section 7: Double Displacement – Gas Producing Reactions - Notes
advertisement

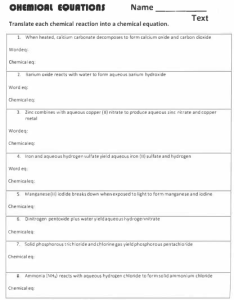
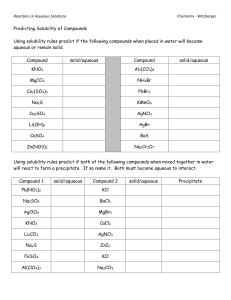
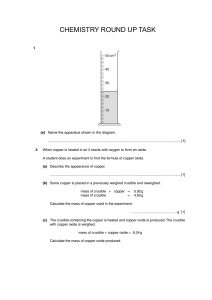
Chapter 6: Chemical Reactions Date:____________ Section 7: Double Displacement – Gas Producing Reactions - Notes Objectives: Identify and write equations for gas evolution reactions. Gas Producing Reactions: Gases that are commonly produced are _____________________________________________, ___________________________________, __________________________________________, ___________________________________, and ______________________________________. o Example: Many gas producing reactions occur when a ___________________________ from a double displacement reaction _____________________________. o Begin by writing a skeletal equation that includes the reactants and products that form when the cation of each reactant combines with the anion of the other. o You must recognize that H2CO3(aq) ___________________________ into ____________ and ________________ and write the corresponding equation. o HNO3(aq) + Na2CO3(aq) → Finally, balance the equation. HNO3(aq) + Na2CO3(aq) → Types of Compounds that Undergo Gas Evolution Reactions H2O(l) + CO2(aq) + NaNO3(aq) Practice: Write chemical, complete ionic, and net ionic equations for each of the following reactions. Perchloric acid reacts with aqueous potassium carbonate, forming carbon dioxide gas and water. Nitric acid reacts with aqueous sodium carbonate. Aqueous hydrobromic acid reacts with aqueous potassium sulfite.