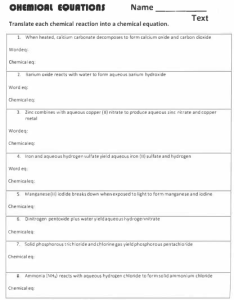
Activity - Chapter 6
advertisement

Class Activity 1. Chapter 6 Balance the following chemical equations a. CaCO3 CaO + CO2 Name b. Na + O2 Na2O c. Mg + N2 Mg3N2 2. d. C2H6 + O2 CO2 + H2O e. PBr3 + H2O HBr + H3PO3 Write a balanced chemical equations from the following word equations. Include the physical state of each of the element or compound a. Sodium metal plus water yields hydrogen gas and an aqueous sodium hydroxide solution b. An aqueous phosphoric acid solution plus an aqueous calcium hydroxide solution yields water and solid calcium phosphate c. Solid phenol (C6H6O) reacts with oxygen to form carbon dioxide gas and liquid water d. Magnesium dissolves in an aqueous chromium (III) nitrate solution to form chromium and a magnesium nitrate solution e. One of the steps in the production of iron involves the reaction of Fe3O4 with carbon monoxide is formed. Write the balanced equation f. Aqueous hydrochloric acid reacts with solid manganese (IV) oxide to form aqueous manganese (II) chloride, liquid water, and chlorine gas.