Atoms Quiz
advertisement

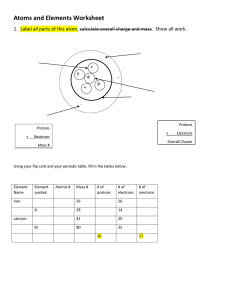
Name: ____________________ Date: _____________ Atomic Structure 1. The 3 particles in an atom are: a.___________________ 2. Their charges are: ____________ 3. Their locations are: ___________________ b.___________________ _____________ ___________________ c. ___________________ ____________ ___________ ______ 4. Use the Periodic Table to give the symbol and number of protons in one atom of: Lithium ________ ________ Bromine ________ ________ Iron Copper ________ ________ ________ ________ Oxygen ________ ________ Mercury ________ ________ Krypton ________ ________ Helium ________ ________ 5. Use the Periodic Table to give the symbol and number of electrons in a neutral atom of: Uranium ________ ________ Chlorine ________ ________ Boron ________ ________ Iodine ________ ________ Antimony ________ ________ Xenon ________ ________ 6. Use the Periodic Table to give the symbol and number of neutrons in a neutral atom of: Potassium ________ ________ Nitrogen ________ ________ Hydrogen ________ ________ Tungsten ________ ________ 7. Define atom. 8. Define Molecule. 8. Define compound. 9. Define mass number, A. 10. Define atomic number, Z. 11. What are the relative masses of a proton, a neutron and an electron? 12. Define Isotopes. 13. State one medical and one industrial use of radioactive isotopes. MCQs 1. Which statement about atoms is correct? A. Atoms contain protons and electrons in the nucleus. B. Neutrons are negatively charged. C. Protons are positively charged. D. The nucleon number is the number of neutrons. 2. What do the nuclei of H 1 1 hydrogen atoms contain? A. electrons and neutrons B. electrons and protons C. neutrons only D. protons only 3. Atoms contain electrons, neutrons and protons. What is the definition of nucleon number? A. the number of neutrons in the nucleus of an atom B. the number of protons in the nucleus of an atom C. the total number of neutrons and protons in the nucleus of an atom D. the total number of particles in an atom 4. What is different for isotopes of the same element? A. nucleon number B. number of electron shells C. number of electrons in the outer shell D. proton number 5. How many atoms of hydrogen are there in a molecule of ethanol, C2H5OH? A. 1 B. 2 C. 5 D. 6 21 Which statement about a neutron is not correct? A It can be present in different numbers in atoms of the same element. B It has no electrical charge. C It is always found in the nucleus of an atom. D It weighs much less than a proton.