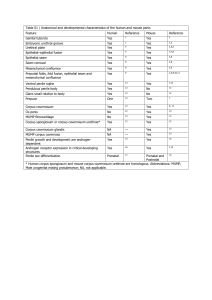
Volume 73, july - august 2013, Issue 4 ISSN-0185
advertisement

Volume 73, july - august 2013, Issue 4 ISSN-0185-4542 ÓRGANO OFICIAL DE DIFUSIÓN DE LA SOCIEDAD MEXICANA DE UROLOGÍA Editor Dr. José Guzmán Esquivel Co-editor Miguel Maldonado Ávila ISSN-0185-4542 * ARTEMISA * SSALUD * LILACS * IMLA * PERIODICA-UNAM * IMBIOMED * LATINDEX Calcified double-J stent management at the Hospital General “Dr. Manuel Gea González” Experience in radical retropubic prostatectomy at the Hospital Regional “Lic. Adolfo López Mateos”, ISSSTE Risk factors for developing urethral stricture in patients that underwent transurethral resection of the prostate Stricture in the bulbous urethra with measurements of length and depth. pp. 180-186 Oncologic effectiveness and safety of laparoscopic renal cryosurgery guided by high definition laparoscopic ultrasound Usefulness of urethral ultrasound imaging in urethral stricture Tumors of Cowper’s glands: a review of the literature A new theory on albumin glomerular filtration and its tubular reabsorption: disputing the charge selectivity theory A) and B) Voiding cystourethrogram that shows stricture data at the penoscrotal junction and the bulbous urethra. C) and D) Urethral ultrasound that shows a reduction in the caliber of the urethra at the level of the penoscrotal junction and the bulbous urethra. Spongiofibrosis surrounding the tissue can be seen. pp. 180-186 www.elsevier.es •CONTENIDO •CONTENTS Artículos originales Manejo de catéteres doble J calcificados en el Hospital General “Dr. Manuel Gea González” ORIGINAL ARTICLES 155 A. J. Camacho-Castro, et al. A. J. Camacho-Castro, et al. Experiencia en prostatectomía radical retropúbica en el Hospital Regional “Lic. Adolfo López Mateos”, ISSSTE 160 P. Cruz García-Villa, et al. 166 P. Cruz García-Villa, et al. 160 Risk factors for developing urethral stricture in patients that underwent transurethral resection of the prostate 166 P. Cruz García-Villa, et al. Eficacia y seguridad oncológica de la criocirugía renal laparoscópica guiada con ultrasonido laparoscópico de alta definición 175 J. G. Campos-Salcedo, et al. Oncologic effectiveness and safety of laparoscopic renal cryosurgery guided by high definition laparoscopic ultrasound 175 J. G. Campos-Salcedo, et al. Utilidad ultrasonido uretral en estenosis de uretra 180 P. Cruz García-Villa, et al. Usefulness of urethral ultrasound imaging in urethral stricture 180 P. Cruz García-Villa, et al. Artículos de revisión Tumores de las glándulas de Cowper: una revisión de la literatura REVIEW ARTICLES 187 A. Lisker-Cervantes, et al. Tumors of Cowper’s glands: a review of the literature 187 A. Lisker-Cervantes, et al. Nueva teoría sobre la filtración glomerular de albúmina y su reabsorción tubular: refutado de la teoría de la “selectividad por cargas” B. Condado-Arenas, et al. Director General: Experience in radical retropubic prostatectomy at the Hospital Regional “Lic. Adolfo López Mateos”, ISSSTE P. Cruz García-Villa, et al. Factores de riesgo para el desarrollo de estenosis de uretra en pacientes operados de resección transuretral de próstata Editada por: Calcified double-J stent management at the Hospital 155 General “Dr. Manuel Gea González” 191 A new theory on albumin glomerular filtration and its tubular reabsorption: disputing the charge selectivity theory B. Condado-Arenas, et al. MASSON DOYMA MÉXICO, SA. Av. Insurgentes Sur 1388, Piso 8, Col. Actipan Del. Benito Juárez, CP 03230, México, D.F. Tels.: 5524-1069, 5524-4920, Fax: 5524-0468. Pedro Turbay Garrido 191 Casos clínicos Esfínter urinario artificial para el manejo de la incontinencia urinaria posterior a prostatectomía radical CLINICAL CASES 195 G. Fernández-Noyola, et al. Ectopia renal cruzada con fusión y litiasis múltiple, nefrectomía con abordaje paramedio anterior 200 Crossed renal ectopia with fusion and multiple renal calculi managed with nephrectomy through the anterior paramedian approach 204 Application of dorsal buccal mucosa graft for penile urethral stricture treatment: technical aspects G. Fernández-Noyola, et al. Manejo de la incontinencia urinaria masculina 208 posprostatectomía radical con cabestrillo transobturador (AdVance®) Management of post-radical prostatectomy male urinary incontinence with a transobturator sling (AdVance®) S. Ahumada-Tamayo, et al. S. Ahumada-Tamayo, et al. 212 Management of recurrent stricture of the perineal meatus with the Blandy technique after penectomy secondary to corpora cavernosa abscess J. Á. Martínez, et al. J. Á. Martínez, et al. Nefrectomía parcial laparoscópica. Aspectos técnicos 216 Technical aspects of laparoscopic partial nephrectomy S. Ahumada-Tamayo, et al. 200 F. R. Zamora-Varela, et al. G. Fernández-Noyola, et al. Técnica de Blandy para el manejo de la estenosis recurrente de meato perineal, posterior a falectomía secundaria a absceso de cuerpos cavernosos 195 G. Fernández-Noyola, et al. F. R. Zamora-Varela, et al. Aplicación de injerto dorsal de mucosa oral para el tratamiento de la estenosis de uretra peniana. Aspectos técnicos Urinary incontinence management with artificial urinary sphincter following radical prostatectomy S. Ahumada-Tamayo, et al. 204 208 212 216 Rev Mex Urol 2013;73(4):155-159 ÓRGANO OFICIAL DE DIFUSIÓN DE LA SOCIEDAD MEXICANA DE UROLOGÍA www.elsevier.es/uromx Original article Calcified double-J stent management at the Hospital General “Dr. Manuel Gea González” A. J. Camacho-Castro*, V. Osornio-Sánchez, J. Á. Martínez, A. Urdiales-Ortiz, G. Fernández-Noyola, S. Ahumada-Tamayo, F. García-Salcido, E. Muñoz-Ibarra, E. MayorgaGómez, G. Garza-Sainz, Z. A. Santana-Ríos, R. Pérez-Becerra, S. Fulda-Graue, C. Martínez-Arroyo, M. Cantellano-Orozco, G. Morales-Montor and C. Pacheco-Gahbler Department of Urology, Hospital General “Dr. Manuel Gea González”, Mexico City, Mexico KEYWORDS Encrustation; Ureteral stent; Calcified; Mexico. Abstract Background: Encrustation is a clinical problem that occurs in both external and internal urinary diversion catheters; the chemical constituents of urine combine with the stent to produce a matrix upon which a stone will later form. Aims: The objective of this article was to describe the management and results obtained in patients with calcified double-J stents at the Hospital General “Dr. Manuel Gea González”. Material and methods: A retrospective, observational, cross-sectional study was carried out. All patients with a calcified ureteral stent at the Urology Service of the Hospital General “Dr. Manuel Gea González” within the time frame of January 2010 to July 2011 were taken into account. They were classified according to the FECal Ureteral Stent Grading System created by the Department of Urology at the Loyola University Medical Center in Maywood, Illinois. Results: Ten patients (5 men and 5 women) presented with calcified double-J stent and their mean age was 46 years. The mean length of time with the indwelling double-J stent was 10.2 months; 4 of the patients were classified as grade II, 2 as grade III, 2 as grade IV, and 2 as grade V. The problem was resolved in 3 of the patients through open surgery, in 3 through laparoscopy, in 3 through endoscopy, and one patient underwent extracorporeal shock wave lithotripsy (ESWL). At present all patients are free from residual stones. Discussion: The management of retained and encrusted ureteral stents can be a surgical challenge for the urologist and represents an increased risk for patient morbidity. However, there are a wide variety of therapeutic options for approaching this pathology. Conclusions: The presence of a classification system and management protocol for calcified ureteral stents enables a standardized approach to this phenomenon. * Corresponding author at: Calzada de Tlalpan N° 4800, Colonia Sección XVI, Delegación Tlalpan, C. P. 14080, México D.F., México. Telephone: 4000 3000, ext. 3298. Email: ajcc7@hotmail.com (A. J. Camacho-Castro). 0185-4542 - see front matter © 2013. Revista Mexicana de Urología. Publicado por Elsevier México. Todos los derechos reservados. 156 Palabras clave Incrustación; Catéter ureteral; Calcificado; México. A. J. Camacho-Castro et al Manejo de catéteres doble J calcificados en el Hospital General “Dr. Manuel Gea González” Resumen Introducción: La incrustación es un problema clínico que ocurre en los catéteres de derivación urinaria tanto externos como internos; los constituyentes químicos de la orina se combinan con el catéter para formar una matriz en donde posteriormente se formará un lito. Objetivo: El objetivo del trabajo fue describir el manejo y los resultados obtenidos en los pacientes con catéter doble J calcificado, en el Hospital General “Dr. Manuel Gea González”. Material y métodos: Estudio retrospectivo, observacional, transversal. Se tomaron en cuenta todos los pacientes con catéter ureteral calcificado, manejados desde enero del 2010 hasta julio del 2011 en el Servicio de Urología, del Hospital General “Dr. Manuel Gea González”. Se clasificaron de acuerdo al FECal Ureteral Stent Grading System, creado por el Departamento de Urología del Centro Médico de la Universidad de Loyola en Maywood, Illinois, EUA. Resultados: Diez pacientes con presencia de catéter doble J calcificado, 5 hombres y 5 mujeres, con una edad promedio de 46 años y tiempo de colocación del catéter doble J de 10.2 meses; 4 se encontraron en grado II, 2 en grado III, 2 en grado IV y 2 en grado V; 3 se resolvieron por medio de cirugía abierta, 3 por laparoscopía, 3 por endoscopía y uno por litotripsia extracorpórea por onda de choque (LEOCH). A la fecha todos los pacientes se encuentran libres de presencia de lito residual. Discusión: El manejo de catéteres ureterales retenidos e incrustados puede representar un reto quirúrgico para el urólogo, constituyendo un riesgo aumentado en la morbilidad del paciente, sin embargo se cuentan con amplias opciones para abordar esta patología. Conclusiones: La presencia de un sistema de clasificación y protocolo de manejo de catéteres ureterales calcificados, permite estandarizar la forma en que se aborda este fenómeno. Introduction The introduction of the ureteral stent in 1967 revolutionized the way urinary tract obstructions were managed and it became one of the most widely used urologic accessories. Current indications for its use include the prevention and treatment of ureteral obstruction secondary to intrinsic, extrinsic, or iatrogenic causes such as urolithiasis, stricture, and malignancy. A calcified ureteral stent is defined as one that cannot be removed by cystoscopy in the first attempt without the aid of other auxiliary measures due to encrustation or the formation of a stone within the stent (fig.1).1-3 Modern ureteral stents have a double-pigtail (double-J) design and are made of synthetic polymers (polyurethane/ polyethylene). The ideal material for a ureteral stent is biocompatible, radiopaque, encrustation-resistant, prevents infection, causes very little discomfort, is economically accessible, and effectively improves the urinary tract obstruction. However, currently no ureteral stent meets all those requirements.4 The majority of polymer-based ureteral stents have a mean indwelling time of 3 to 6 months. Current advances in the stents are focused on preventing symptoms or complications associated with their placement, such as infection, migration, dysuria, and calcification.5,6 These advances may reduce the desire of the patient to have the stent removed thanks to a decrease in the related symptomatology and these data, together with an increase in stent use, can be extrapolated to an increase in future calcified double-J stents.7-9 In March 2009 the Department of Urology at the Loyola University Medical Center in Maywood, Illinois published the FECal Ureteral Stent Grading System, along with a management protocol that enable cases to be resolved with the most effective methods depending on the classification grade of the stent10. The aim of the present study was to describe the management and results obtained in patients with a calcified double-J stent seen at the Hospital General “Dr. Manuel Gea González”. Methods A descriptive, retrospective, observational and cross-sectional study was carried out. All the patients presenting with a calcified ureteral stent that were managed within the time frame of January 2010 to July 2011 at the Urology Service of Figure 1 Encrustation. Calcified double-J stent management at the Hospital General “Dr. Manuel Gea González” 157 Table 1 Management by calcification grades Grade N Management I 0 NA II 4 Endoscopic III 2 Open/endoscopic IV 2 Open V 2 Laparoscopic/endoscopic Grade IGrade IIGrade IIIGrade IVGrade V Figure 2 Calcification grades. the Hospital General “Dr. Manuel Gea González” were included in the study. The stents were classified according to the FECal ureteral stent grading system (fig. 2) as follows: • Grade I: Minimal linear encrustation at either of the pigtail loops • Grade II: Circular encrustation that completely encloses either of the pigtail loops • Grade III: Circular encrustation that completely encloses either of the pigtail loops, with some linear encrustation along the ureteral portion of the stent • Grade IV: Circular encrustation that completely encloses both of the pigtail loops • Grade V: Diffuse and bulky encrustation that completely encloses both of the pigtail loops, as well as the entire ureteral portion of the stent Results A total of 92 double-J stents were placed during the abovementioned time frame, of which 10 patients (10.86%) Figure 3 Radiologic calcification grades. presented with calcified double-J stent. Those patients included 5 men and 5 women and their mean age was 46 years. The mean catheter indwelling time was 10.2 months and according to the FECal ureteral stent grading system, 4 of the stents were grade II, 2 were grade III, 2 were grade IV, and 2 were grade V (fig.3); 3 of the calcified stents were resolved through open surgery, 3 through laparoscopy, and one through ESWL (table 1). Only one patient had a failed first attempt that was later resolved through ESWL. At present all of the patients are free from residual stone. The differences in the management of the calcified double-J stents between the protocol suggested by the Medical Center in Maywood and our institution are the approach used for the proximal pigtail loop in grades II, III, and IV, as well as in the grade V complete stent calcification. The suggested protocol is Holmium laser, ESWL, or percutaneous nephrolithotomy (PNL), all of which we substituted with the laparoscopic approach (fig. 4), given that holmium laser was not available at that time at our hospital. Even so, the results were satisfactory and there was complete resolution in the first procedure in 90% of the patients. 158 A. J. Camacho-Castro et al Figure 4 Endoscopic and laparoscopic management. Discussion Conflict of interest Retained and encrusted ureteral stent management can be a surgical challenge for the urologist, as well as an increased risk for patient morbidity. However, there are many options for approaching this pathology that include open surgery, laparoscopy, the percutaneous approach, and endoscopy with lithotripsy (hydraulic and laser). Taking into account that the patient sample obtained in our hospital was smaller that those reported in the medical literature, we had pathology resolution with a single procedure in 90% of our cases, as opposed to the 80% reported in other hospitals worldwide. The complications associated with calcified double-J stent include infections, stent fracture, ureteral obstruction, and loss of renal function. The authors declare that there was no conflict of interest. Conclusions Having a classification system of and management protocol for calcified ureteral stents enables a standardized approach to this phenomenon. However, due to the limited access to all the management options in the different institutions, the treatment plan to follow should be individualized for each patient. Financial disclosure No financial support was received in relation to this article. References 1. Lam JS, Mantu MG. Tips and Tricks for the Management of Retained Ureteral Stents. J Endourol 2002;16(10):733-741. 2. Bukkapatnam R, Seigne J, Helal M. 1-Step Removal of Encrusted Retained Ureteral Stents. J Urol 2003;170:111-1114. 3. Canales BK, Higgins LA. Presence of Five Conditioning Film Proteins Are Highly Associated with Early Stent Encrustation. J Endourol 2009;23(9):1437-1442. 4. Majid Rana A, Sabooh A. Management Strategies and Results for Severely Encrusted Retained Ureteral Stents. J Endourol 2007;21(6):628-632. 5. Chin-Chung Y, Chieh-Hsiao C. A New Technique for Treating Forgotten Indwelling Ureteral Stents: Silk Loop Assisted Ureterorenoscopic Lithotripsy. J Urol 2004;171:719-721. 6. Cass AS, Kavaney P. Extracorporeal Shock Wave Lithotripsy for Calcified Ureteral Stent. J Endourol 1993;7(1):7-10. Calcified double-J stent management at the Hospital General “Dr. Manuel Gea González” 7. Monga M, Klein E, Castañeda-Zuñiga WR, et al. The Forgotten Indwelling Ureteral Stent: A Urological Dilemma. J Urol 1995;153:1817-1819. 8. Aravantinos E, Gravas S. Forgotten, Encrusted Ureteral Stents: A Challenging Problem with an Endourologic Solution. J Endourol 2006;20(2):1045-1049. 159 9. Vanderbrink BA, Rastinehad A.R. Encrusted Urinary Stents: Evaluation and Endourologic Management. J Endourol 2008;22(5):905-912. 10. Acosta-Miranda AM, Miner J. The FECal Double-J: A Simplified Approach in the Management of Encrusted and Retained Ureteral Stents. J Endourol 2009;15(3):409-415. Rev Mex Urol 2013;73(4):160-165 ÓRGANO OFICIAL DE DIFUSIÓN DE LA SOCIEDAD MEXICANA DE UROLOGÍA www.elsevier.es/uromx Original article Experience in radical retropubic prostatectomy at the Hospital Regional “Lic. Adolfo López Mateos”, ISSSTE P. Cruz García-Villa*, M. Estrada-Loyo, D. López-Alvarado and E. Monroy-Bolaños Urology Speciality Residency, Hospital Regional “Lic. Adolfo López Mateos”, ISSSTE, Mexico City, Mexico KEYWORDS Radical prostatectomy; Prostate cancer; Complications; Mexico. Experience in radical retropubic prostatectomy at the Hospital Regional “Lic. Adolfo López Mateos”, ISSSTE Abstract Background: Radical retropubic prostatectomy (RRP) is the curative surgical treatment for prostate cancer (CaP). When performing this complex surgery, the intention is to diminish the possibility of urinary incontinence and erectile dysfunction. Aims: To describe the experience of patients that underwent RRP at our hospital. Material and methods: A descriptive, cross-sectional, retrospective study was conducted on patients that underwent RRP within the time frame of 2008 to 2011 at the Hospital Regional Lic. Adolfo López Mateos of the ISSSTE in Mexico City. Results: A total of 38 radical prostatectomies were performed. The mean age was 60 years. The mean prostate specific antigen (PSA) value at the time of diagnosis was 10.2 ng/ml and the mean Gleason score obtained through transrectal ultrasound (TRUS)-guided prostate biopsy was 5.6. The previous clinical stage was T1c in 68.4% of the patients and the definitive histopathologic study was positive in 71.1%; the surgical margins were positive in 15.8% of the cases and 45% of the patients presented with erectile dysfunction. Discussion: RRP as a cure requires experience and is not free from complications. The demographic, clinical, surgical, and morbidity data of our study were similar to those of other authors. Conclusions: RRP continues to be the treatment of choice in confined CaP. This procedure has specific morbidity and mortality. However, because of its curative potential, it is the most beneficial option in well-selected patients. * Corresponding author at: Av. Universidad N° 1321, Colonia Florida, Delegación Álvaro Obregón, C.P. 01030, México D.F., México. Telephone: 5322 2300. Email: patricio_cruzgar@yahoo.com.mx (P. Cruz García-Villa). 0185-4542 - see front matter © 2013. Revista Mexicana de Urología. Publicado por Elsevier México. Todos los derechos reservados. Radical prostatectomy at the Hospital López Mateos, ISSSTE Palabras clave Prostatectomía radical; Cáncer de próstata; Complicaciones; México. 161 Experiencia en prostatectomía radical retropúbica en el Hospital Regional “Lic. Adolfo López Mateos”, ISSSTE Resumen Introducción: El tratamiento quirúrgico curativo para el cáncer de próstata (CaP) es la prostatectomía radical retropúbica (PRR). Se intenta disminuir la posibilidad de incontinencia urinaria y disfunción eréctil. La PRR es una cirugía compleja. Objetivo: Describir la experiencia en PRR, en pacientes operados en nuestro Hospital. Material y métodos: Se realizó un estudio descriptivo, transversal y retrospectivo de los pacientes sometidos a PRR de 2008 a 2011, en el Hospital Regional “Lic. Adolfo López Mateos” del ISSSTE, México D.F. Resultados: Se realizaron 38 prostatectomías radicales. La edad promedio fue de 60 años. El antígeno prostático específico (APE) medio al diagnóstico fue de 10.2 ng/mL y la suma de Gleason por biopsia transrectal de la próstata (BTRP) fue de 5.6. El estadio clínico previo correspondía a T1c en el 68.4%. El estudio histopatológico definitivo fue positivo en el 71.1%. En el 15.8% los márgenes fueron positivos. El 45% presentó disfunción eréctil. Discusión: La PRR como tratamiento curativo requiere experiencia y no está libre de complicaciones. De acuerdo este estudio, encontramos datos demográficos, clínicos, quirúrgicos, y de morbilidad similares a los de otros autores. Conclusión: La PRR continúa siendo el tratamiento de elección en CaP confinado. Este procedimiento tiene morbilidad y mortalidad específica. Sin embargo, el ser potencialmente curativo lo hace la opción de más valor en pacientes bien seleccionados. Introduction Prostate cancer (CaP) is the number two disease in regard to mortality burden in the United States, just behind lung cancer, and it is the most common non-dermatologic tumor affecting men in the Western world; radical prostatectomy (RP) is the standard treatment in the majority of patients with recently diagnosed, clinically localized disease, with close to 50% of them undergoing surgery.1 The ideal result of RP is for the patient to have undetectable prostate-specific antigen (PSA) levels and no functional consequences.2 A critical component in the evaluation of oncologic effectiveness in the case series that have been published - and that can be used in comparative analyses - is to be able to measure the result that is going to be reported. At the lowest level, establishing the measurement of the result of interest becomes vitally importance for being able to answer the question patients most frequently ask: “What is the surgery’s expected range of success?” Up to the present, the most commonly reported measurement of cancer control after RP has been biochemical recurrence (PSA level).3 Whereas the principal aim of the surgery is complete removal of the primary tumor, patient satisfaction can be negatively affected by the resulting postoperative urinary incontinence and/or erectile dysfunction. Therefore, it is necessary to include both the oncologic and the functional results in evaluating success after RP. In an attempt to improve the evaluation of RP results, the addition of postoperative complications and the status of the surgical specimen margins have been proposed.1 The report on a large group of patients with CaP that have undergone RP, their postoperative complications, biochemical recurrence, and erectile function and urinary incontinence statuses is of great importance and should be carried out both inside and outside the medical institutions so that comparisons among them can be established. Such an analysis would result in the implementation of improved surgical techniques and the strengthening of areas that require it. Methods A descriptive, analytic, cross-sectional, and retrospective study was conducted on patients with CaP that received curative surgical treatment (radical retropubic prostatectomy, RRP) within the time frame of 2008 to 2011 at the Hospital Regional Lic. Adolfo López Mateos of the ISSSTE. The following variables were analyzed: age, comorbidities, diagnostic PSA, Gleason score from biopsy, clinical stage, prior use of androgen blockade, intraoperative blood loss, surgery duration, hospital stay, definitive histopathologic result, positive surgical margins, seminal vesicles, lymph nodes, control PSA at 3, 6, and 12 months, biochemical recurrence rate, adjuvant treatment, urinary incontinence incidence at 6 months, erectile dysfunction at 6 months, and other complications. Means, standard deviation, and data frequency were reported. The paired Student’s t test was used to compare the Gleason score from the TRUS-guided prostate biopsy with the definitive Gleason score. Results In the period corresponding to the years 2008, 2009, 2010, and 2011, 38 open RRPs were registered. The mean age of the patients was 60.89 ± 4.8 years (range: 49-69 years). A total of 15.8% of the patients had a past history of diabetes mellitus type 2 (DM2) and 31.6% of high blood pressure. The 162 P. Cruz García-Villa et al Table 1 Demographic characteristics of the patients included in the study Age (years) Diabetes mellitus type 2 60.89 ± 4.8 15.8% High blood pressure 31.6% Initial PSA (ng/mL) 10.2 ± 6.2 Total Gleason score of the TRUS-guided prostate biopsy 5.6 3+3 36.8% 2+2 21.1% 3+4 15.8% 4+3 7.9% Preoperative clinical stage T1c 68% T1b 7.9% T2a 18% T2b 5.3% Total Gleason score of the surgical specimen 6.3 Surgery duration (minutes) 257 Intraoperative blood loss (mL) 2000 Tumor-free margins 84.2% Vesicle infiltration 15.8% Hospital stay (days) 6.9 ± 4 Urinary continence (6 months) 75% Erectile dysfunction 45% p<0.005 PSA: prostate-specific antigen; TRUS: transrectal ultrasound. mean PSA with which the TRUS-guided prostate biopsy was carried out was 10.2 ± 6.2 ng/mL. The mean TRUS-guided prostate biopsy result showed a Gleason score of 5.6; 36.8% of the patients had 3+3, 21.1% had 2+2, 15.8% had 3+4, and 7.9% had 4+3. Clinical stage corresponded to T1c in 68% of the patients, T2a in 18%, T1b in 7.9%, and T2b in 5.3%. Androgen deprivation therapy was administered to 50% of the patients at some point before surgery. In relation to the surgical procedure, the mean surgery duration was 257 minutes (range: 150-480 minutes), with a mean blood loss of 2,000 mL (range: 350-5,000 mL). All patients underwent bilateral pelvic lymph node dissection. The mean hospital stay was 6.9 ± 4 days. The definitive histopathologic result was positive in 71% (n=27) of the patients. Immunohistochemistry with p63 and p504 was carried out in 2 patients for the definitive diagnosis. The mean Gleason score result was 6.3. The paired Student’s t test was used to compare the Gleason score from the TRUS-guided prostate biopsy and the surgical specimen and there was a significant difference (p<0.005) (table 1). Only one patient presented with lymph node metastasis in the definitive histopathologic study. Tumor-free margins were reported in 84.2% (n=32) of the patients and tumor infiltration into the seminal vesicles was reported in 15.8%. In 25 patients the mean PSA at 3 months was .86 ng/mL (range: 0.0 to 8.1). At 6 and 12 months, 37 patients had a mean control PSA of .62 ng/mL (0.0-8.6) and .77 ng/mL (0.0-17), respectively. There was biochemical recurrence at some point in 60% of the patients with a PSA of 0.04 ng/mL as the minimum range. Androgen bock was begun in 37.8% of the patients at some point after surgery due to biochemical recurrence. A total of 21% (n=8) patients had a histopathologic report of positive margins or seminal vesicle infiltration, indicating adjuvant radiotherapy. Some degree of urinary incontinence was present in 25% of the patients at 6 months after surgery, whereas 45.7% presented with erectile dysfunction in the first semester after the procedure. Discussion Despite the emergence of new therapeutic forms for organconfined CaP, RP continues to be the only curative surgical procedure. Even though it is a technique that has been perfected over time, this procedure is still associated with mortality and morbidity.4-14 Adequate cancer staging, the general conditions of the patient, the surgical technique, and the experience of the surgeon or surgeons are factors that directly affect the success of the RRP. Currently there are also minimally invasive approaches such as laparoscopic surgery and the more recent robot-assisted surgery. Although these procedures offer advantages such as less blood loss, better visualization, shorter surgery duration, shorter hospital stay, and a lower rate of certain complications, many urology surgeons continue to perform open retropubic surgery with similar results.15 RP was first described by Young in 1901, and in 1947 Millen depicted the retropubic approach. In 1982, Walsh et al. described the technique of nerve-sparing RRP.16 Also known as nerve-sparing prostatectomy, this technique consists of the extirpation of the prostate gland with early hemostatic control for achieving good visualization of the urethral sphincter, as well as the nerve bundles that innervate the corpora cavernosa. According to Walsh, erection and urinary continence can be preserved with this technique in the majority of patients and it has a reported surgical mortality of 0.5%.17-18 Walsh reported potency rates of 68% and continence rates of 92%. The technique described by Walsh is the one that is currently performed in the majority of centers carrying out this type of surgery. In our institution, residents are trained based on the principles of the technique described by Walsh. In our study population, the mean age was 60 years and the mean PSA was 10.2 ± 6.2 ng/mL. The tumors had a mean TRUS-guided prostate biopsy Gleason score of 5.6 and tumor stage was T1c in 68%, T2a in 18%, T1b in 7.9%, and T2b in 5.3%. In the comparison of our data with other studies, the results were similar in relation to age, PSA, TRUS-guided prostate biopsy Gleason score, and clinical stage at the time of diagnosis.6,20-29 The postoperative Gleason score was higher in relation to that obtained through the TRUS-guided prostate biopsy and the difference was statistically significant. Just as in other studies, the TRUS-guided prostate Radical prostatectomy at the Hospital López Mateos, ISSSTE Table 2 Positive margin comparison following radical prostatectomy Author Year No. Positive margin % Ward20 2004 7,268 2,772 (38%) Pettus21 2004 498 98 (20%) Han22 2004 9,035 1,324 (14%) Swindle23 2005 1,389 179 (13%) Karakiewicz24 2005 5,831 1,554 (27%) Simon 2006 936 2006 281 2007 2,242 275 (11%) 2008-2011 38 6 (16%) 25 Vis26 Eastham 27 Cruz et al. 163 Table 3 Comparison of different studies showing the urinary continence percentages at one year after radical prostatectomy. Continence % 6 months Continence % 12 months Hammerer y Hulland 85 91 Walsh et al. 80 93 Nandipati et al. 70 80 Lepor y Kaci 87 92 Stanford et al. 83 89 350 (37%) Donellan et al. 72 84 66 (23.5%) Cruz et al. 75 - biopsy understaged the tumors, and higher Gleason scores were obtained from the surgical specimen. RRP is regarded as a difficult and highly complex surgery whose oncologic and functional results depend on the technique. Due to the location of the prostate gland and the vascular and nervous structures that surround it, RRP surgery is prone to the development of intraoperative and postoperative complications. In a descriptive study of 1,000 RRPs, the most frequent intraoperative complication was rectal injury. The most common immediate postoperative complication was acute myocardial infarction, followed by pulmonary thromboembolism and excessive blood loss. Of the late postoperative complications, bladder neck stricture was the most frequent, followed by seroma in the wound, and acute urine retention. The causes of reintervention were hemorrhage in 0.3% of the patients and anastomosis failure in 0.2%. Hospital stay was 2.3 days and in our case series it was 6.9 ± 4 days.6 As with any oncologic principle, complete tumor extraction is a priority over function. The presence of malignant cells on the surgical edge of the specimen means incomplete resection of the prostate tumor. This confers a poor outcome on the patient because the disease has now spread beyond the limits of the prostate gland. Unfortunately, positive surgical margins signify the failure of a treatment that was intended to be curative. Despite the advances in the technique, positive margins in the pathology specimen are not uncommon and this represents a greater risk for biochemical recurrence and systemic disease. A positive margin can be described as a tumor that extends to the stained surface of the prostatectomy specimen.29 Positive margins can be classified as iatrogenic and non-iatrogenic. The former are those in which there was disruption of the capsule during the surgery and therefore part of the gland was not extracted.27 According to the reviewed data, there was a 16% positive margin rate in our population. In the comparison with other authors, we found the incidence of positive margins to range from 11% to 38% (table 2). References Urinary incontinence is one of the complications that most affects the quality of life of the patients after a RP. The complex that forms the urethral sphincter is responsible for urinary continence. Due to the proximity of the prostatic apex, the sphincteric mechanism can be injured. Urinary incontinence following radical prostate surgery is defined as the involuntary exit of urine, preceded or not by the accompanying sensation. This incontinence is generally stress incontinence, and after surgery, with time, the sphincteric function can be recovered, even up to 24 months later. Even though it is difficult to measure the degree of incontinence, in general an adequate manner is asking how many protectors a patient needs to use daily. Another option is to apply a validated questionnaire. According to the collected data of our patients, 75% of them were continent at 6 months. Unfortunately the follow-up of some patients was lost, making it impossible to determine that rate at 12 months. Loughlin and Prasad carried out a review of various studies and found continence rates at 6 months that ranged from 70% to 87% and that showed improvement at 12 months30 (table 3). On the other hand, erectile dysfunction is defined as the inability to achieve or maintain a penile erection that is sufficient for penetration. As a consequence of the manipulation and excision of nerve fibers that innervate the corpora cavernosa, erectile dysfunction can become present or worsen after radical retropubic surgery. According to studies, the percentage of erectile dysfunction after surgery varies from 25% to 75%.31-33 In our study, the erectile dysfunction after surgery was 45.7% in the first semester. It should be mentioned that erectile function before surgery was not evaluated in our patients and so we cannot guarantee that these results are due completely to the prostatectomy in all of the patients. The use of 5-phosphodiesterase inhibitors is well documented for early rehabilitation of erectile function in this group of patients, accelerating recovery as well as quality of the erection. Although RRP is regarded and performed as a curative method, these patients are not exempt from presenting with biochemical recurrence with the passage of time. Some authors consider biochemical recurrence in patients 164 that have undergone RRP to be a PSA level greater than 0.04 ng/mL, while others use the value of 0.02 ng/mL. The expected PSA value after surgery is zero. In the group of patients operated on at our institution, 60% presented with PSA values above 0.04 ng/mL at some point after surgery, and so were regarded as presenting with biochemical failure. Of those patients, 37.8% were given androgen deprivation therapy. Conclusions RRP is a surgery that should be performed on well-selected patients presenting with organ-confined prostate cancer. Both the urologist and patient should analyze the cost/benefit of the surgery, as well as its risks and benefits. We believe it is necessary to learn the technique of RRP, given that it continues to be the recommended procedure in comparison with laparoscopic or robot-assisted surgery. Not only the early diagnosis of CaP, but also the mastery of the surgical technique is important for achieving a low incidence of complications and greater curative success. The knowledge of institutional data enables the recognition of what was done correctly and what was not, for the sole purpose of improving the success and cure rates with the least number of complications for the patient. Conflict of interest The authors declare that there was no conflict of interest. Financial disclosure No financial support was received in relation to this article. References 1. Ficarra V, Sooriakumaran P, Novara G, et al. Systematic Review of Methods for Reporting Combined Outcomes After Radical Prostatectomy and Proposal of a Novel System: The Survival, Continence, and Potency (SCP) Classification. Eur Urol 2012;61(3):541-548. 2. Boorjian S, Eastham J, Graefen M, et al. A Critical Analysis of the Long-Term Impact of Radical Prostatectomy on Cancer Control and Function Outcomes. Eur Urol 2012;61(4):664-675. 3. Dahl D, Barry M, McGovern F, et al. Prospective Study of Symptom Distress and Return to Baseline Function After Open Versus Laparoscopic Radical Prostatectomy. J Urol 2009;182(3):956965. 4. Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol 1998;160(6 Pt 2):2418-2424. 5. Steiner MS. Continence-preserving anatomical radical retropubic prostatectomy. Urology 2000;55(3):427-435. 6. Lepor H, Nieder AM, Ferrandino MN. Intraoperative and postoperative complications of radical retropubic prostatectomy in a consecutive series of 1,000 cases. J Urol 2001;166(5):17291733. 7. Dillioglugil, O, Leibman BD, Leibman NS, et al. Risk factors for complications and morbidity after radical retropubic prostatectomy. J Urol 1997;157(5):1760-1767. P. Cruz García-Villa et al 8. Hautmann RE, Sauter TW, Wenderoth U. Radical retropubic prostatectomy: morbidity and urinary incontinence in 418 consecutive cases. Urology 1994;43(2 Suppl):47-51. 9. Hammerer P, Hubner D, Gonnermann D, et al. Perioperative and postoperative complications in pelvic lymphadenectomy and radical prostatectomy in 320 consecutive patients. Urologe A 1995;34(4):334-342. 10. Davidson PJ, Van den Ouden D, Schroeder FH. Radical prostatectomy: prospective assessment of mortality and morbidity. Eur Urol 1996;29(2):168-173. 11. Gaylis FD, Friedel WE, Armas OA. Radical retropubic prostatectomy outcomes at a community hospital. J Urol 1998;159(1):167-171. 12. Catalona WJ, Carvalhal GF, Mager DE, et al. Potency, continence and complication rates in 1,870 consecutive radical retropubic prostatectomies. J Urol 1999;162(2):433-438. 13. Gheiler EL, Lovisolo JA, Tiguert R, et al. Results of a clinical pathway for radical prostatectomy patients in an open hospital-multiphysician system. Eur Urol 1999;35(3):210-216. 14. Arai Y, Egawa S, Tobisu K, et al. Radical retropubic prostatectomy: time trends, morbidity and mortality in Japan. BJU Int 2000;85(3):287-294. 15. Rassweiler J, Othmar S, Schulze M. Laparoscopic versus open radical prostatectomy: A comparative study at a single institution. J Urol 2003;169(5):1689-1693. 16. Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol 1982;128(3):492-497. 17. Steiner MS, Morton RA, Walsh PC. Impact of anatomical radical prostatectomy on urinary continence. J Urol 1991;145(3):512-514. 18. Walsh PC, Partin AW, Epstein JI. Cancer control and quality of life following anatomical radical retropubic prostatectomy: results at 10 years. J Urol 1994;152(5 Pt 2):1831-1836. 19. Ward JF, Zincke H, Bergstralh EJ, et al. The impact of surgical approach (nerve bundle preservation versus wide local excision) on surgical margins and biochemical recurrence following radical prostatectomy. J Urol 2004;172(4 Pt 1):1328-1332. 20. Pettus JA, Weight CJ, Thompson CJ, et al. Biochemical failure in men following radical retropubic prostatectomy: impact of surgical margin status and location. J Urol 2004;172(1):129132. 21. Han M, Partin AW, Chan DY, et al. An evaluation of the decreasing incidence of positive surgical margins in a large retropubic prostatectomy series. J Urol 2004;171(1):23-26. 22. Swindle P, Eastham JA, Ohori M, et al. Do margins matter? The prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol 2005;174(3):903-907. 23. Karakiewicz PI, Eastham JA, Graefen M, et al. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology 2005;66(6):1245-1250. 24. Simon MA, Kim S, Soloway MS. Prostate specific antigen recurrence rates are low after radical retropubic prostatectomy and positive margins. J Urol 2006;175(1):140-144. 25. Vis AN, Schroder FH, Van der Kwast TH. The actual value of the surgical margin status as a predictor of disease progression in men with early prostate cancer. Eur Urol 2006;50(2):258-265. 26. Eastham JA, Kuroiwa K, Ohori M, et al. Prognostic significance of location of positive margins in radical prostatectomy specimens. Urology 2007;70(5):965-969. 27. Yossepowitch O, Bjartell A, Eastham J, et al. Positive surgical margins in radical prostatectomy: outlining the problem and its long-term consequences. Eur Urol 2009;55(1):87-99. 28. Mullins JK, Han M, Pierorazio PM, et al. Radical Prostatectomy outcome in Men 65 Years Old or Older With Low Risk Prostate Cancer. J Urol 2012;187(5):1620-1625. Radical prostatectomy at the Hospital López Mateos, ISSSTE 29. Epstein JI, Amin M, Boccon-Gibod L, et al. Prognostic factors and reporting of prostate carcinoma in radical prostatectomy and pelvic lymphadenectomy specimens. Scand J Urol Nephrol Suppl 2005;(216):34-63. 30. Loughlin KR, Prassad MM. Post-Prostatectomy Urinary Incontinence: A Confluence of 3 Factors. J Urol 2010;183(3):871-877. 31. Mirone V, Imbimbo C, Palmieri A, et al. Erectile dysfunction after surgical treatment. Int J Androl 2003;26(3):137-140. 165 32. Robinson JW, Moritz S, Fung T. Meta-analysis of rates of erectile function after treatment of localized prostate carcinoma. Int J Radiat Oncol Biol Phys 2002;54(4):1063-1068. 33. Vale J. Erectile dysfunction following radical therapy for prostate cancer. Radiother Oncol 2000;57(3):301-305. Rev Mex Urol 2013;73(4):166-174 ÓRGANO OFICIAL DE DIFUSIÓN DE LA SOCIEDAD MEXICANA DE UROLOGÍA www.elsevier.es/uromx Original article Risk factors for developing urethral stricture in patients that underwent transurethral resection of the prostate P. Cruz García-Villaa,*, M. Schroede-Ugaldea, M. Landa Soler-Martínb and F. Mendoza-Peñac a Urology Speciality Residency, Hospital Regional “Lic. Adolfo López Mateos”, ISSSTE, Mexico City, Mexico b Department of Urology, Hospital Regional “Lic. Adolfo López Mateos”, ISSSTE, Mexico City, Mexico c Department of Urology Administration, Hospital Regional “Lic. Adolfo López Mateos”, ISSSTE, Mexico City, Mexico KEYWORDS Stricture; Urethra; Transurethral resection of the prostate; Mexico. Abstract Background: Transurethral resection of the prostate (TURP) is currently one of the most widely used treatments for managing prostatic hyperplasia. One of the risks of this procedure is the formation of urethral stricture, defined as a narrowing of the urethral lumen secondary to cicatrization. Different factors intervene in the formation of urethral narrowings in patients that undergo TURP. Aims: To determine the risk factors for post-TURP urethral stricture. Results: In accordance with the established criteria, a total of 63 patients were included in the study; 30 belonged to the group that developed stricture (group A) and 33 belonged to the group that did not (group B). The International Prostate Symptom Score (IPSS) was applied prior to the TURP; group A had a mean score of 19.03 ± 3.78 points and group B of 19.48 ± 5.42. The mean postoperative IPSS for group A was 16.27 ± 5.12 points and for group B was 8.88 ± 4.20 points. A total of 36.7% of the patients that developed stricture had preoperative Foley catheter placement, whereas 69.7% of the patients that did not develop stricture had a catheter at some point prior to surgery (p<0.005). Mean surgery duration for group A was 57.17 ± 17.74 minutes vs. 57.12 ± 20.04 minutes for group B. In group A, surgery lasted more than 60 minutes in 60% of the patients (n=18) and was under 60 minutes in 40% (n=12). In group B, surgery duration was over 60 minutes in 42.4% (n=14) of the patients and under 60 minutes in 57.6% (n=19). In the patients presenting with stricture, the transurethral Foley catheter remained in place after TURP for 8.90 ± 3.91 days vs. 5.15 ± 3.0 days in the patients with no stricture (p<0.05). Conclusions: The principal risk factors for urethral stricture formation in patients that underwent TURP were a prostate volume greater than 80 g determined through transabdominal or transrectal ultrasound prior to surgery, urethral dilation immediately prior to the procedure, resection duration greater than 60 minutes, and the prolonged use of a transurethral catheter following surgery (8.9 ± 3.91 days). * Corresponding author at: Av. Universidad N° 1321, Colonia Florida, Delegación Álvaro Obregón, C.P. 01030, México D.F., México. Telephone: 5322 2300. Email: patricio_cruzgar@yahoo.com.mx (P. Cruz García-Villa). 0185-4542 - see front matter © 2013. Revista Mexicana de Urología. Publicado por Elsevier México. Todos los derechos reservados. Risk factors for posterior urethral stricture following TURP Palabras clave Estenosis; Uretra; Resección transuretral de la próstata; México. 167 Factores de riesgo para el desarrollo de estenosis de uretra en pacientes operados de resección transuretral de próstata Resumen Introducción: La resección transuretral de la próstata (RTUP) es uno de los tratamientos más utilizados actualmente, para el manejo de la hiperplasia prostática. Este procedimiento conlleva riesgos, entre ellos la formación de estenosis de uretra. La estenosis de uretra se define como una estrechez de la luz uretral, secundaria a la formación de una cicatriz. Existen diferentes factores que intervienen en la formación de estrecheces uretrales, en pacientes operados de RTUP. Objetivo: El objetivo fue determinar los factores de riesgo para estenosis uretral post-RTUP. Resultados: De acuerdo a los criterios establecidos, se incluyeron un total de 63 pacientes en el estudio. De éstos, 30 pertenecen al grupo que desarrolló estenosis (grupo A) y 33 al grupo que no desarrolló estenosis (grupo B). La escala internacional de síntomas prostáticos (IPSS, por sus siglas en inglés), previo a la RTUP para el grupo A fue de 19.03 ± 3.78 puntos, y para el grupo B de 19.48 ± 5.42. Por otra parte, el IPSS posquirúrgico para el grupo A fue de 16.27 ± 5.12 puntos, y para el grupo B de 8.88 ± 4.20 puntos. El uso de sonda Foley previo a la cirugía prostática en aquellos que desarrollaron estenosis de uretra fue de 36.7%, mientras que en el grupo que no desarrolló estenosis, un 69.7% portó sonda en algún momento previo a la cirugía (p<0.005). El tiempo quirúrgico para el grupo A fue de 57.17 ± 17.74 minutos vs. 57.12 ± 20.04 minutos para el grupo B. En el grupo A, el 60% (n=18) tuvo una duración mayor de 60 minutos y el 40% menor a 60 minutos, mientras que en el grupo B el 42.4% (n=14) tuvo una duración mayor a 60 minutos y en el 57.6% (n=19) menor a 60 minutos. El tiempo de permanencia de la sonda Foley transuretral posterior a la RTUP, en el grupo de pacientes con estenosis fue de 8.90 ± 3.91 vs. 5.15 ± 3.0 días (p<0.05). Conclusiones: Los principales factores de riesgo para la formación de estenosis uretral en pacientes operados de RTUP son: la presencia de un volumen prostático por ultrasonido trans-abdominal o transrectal previo a la cirugía mayor de 80 g, la realización de dilatación inmediatamente previa al procedimiento, un tiempo de resección mayor a 60 minutos y un uso prolongado de sonda transuretral posterior a la cirugía (8.9 ± 3.91 días). Introduction Benign prostatic hyperplasia (BPH) is currently one of the most frequent health problems affecting the adult male population. It is estimated that 10% of men present with BPH at 30 years of age, 20% at 40 years, 50-60% at 60 years and 80% to 90% at 70 and 80 years of age.1 BPH is the result of the proliferation of fibroblasts, myofibroblasts, and glandular epithelial elements near the urethra in the transitional zone of the prostate.2-5 An enlarged prostate is found in only some of the men presenting with urinary symptoms. Taking into account that the normal size of the prostate is from 20 to 30 mL in the young adult, it has been established that a volume greater than 30 mL represents clinical prostatic hyperplasia.6 Currently, the International Prostate Symptom Score (IPSS) is used for clinical management in patients with lower urinary tract symptoms. Some of the other instruments that are employed are the hyperactive bladder symptom scale, the urinary perception score, and the lower urinary tract symptom result score (table 1).7,8 The clinical diagnosis of BPH is made through obtaining the clinical history from the patient and carrying out the complete medical interview and physical examination. Studies such as ultrasound imaging enable a more precise prostatic volume to be established. Cystourethroscopy has been shown to be less precise in determining the size of the prostate gland. Nevertheless, the shape of the prostate gland can be determined through this type of procedure, and its macroscopic aspect, in accordance with studies carried out by Randall in 1931, can be established.9,10 Treatment for prostatic hyperplasia is medical or surgical. Recurrent urinary tract infections, bladder lithiasis, acute urine retention, symptomatology that is not resolved through medical management, bladder diverticula secondary to chronic prostatic obstruction, hematuria of prostatic origin, and elevated serum creatinine and urea due to prostatic obstruction are some of the indications for surgical management. Transurethral resection of the prostate (TURP) is one of the most widely used surgical treatments worldwide and is considered to be the treatment of choice when drug therapy has not resolved the symptoms. Despite its being performed routinely, this procedure is not free from complications and they can be divided into intraoperative and postoperative ones. The intraoperative complications are blood loss, post-TURP syndrome, extravasation, and ureteral meatus injury. There are early and late postoperative complications. An early complication is bladder tamponade due to heavy clot formation. Infection is rare, although one study reported its presence in 21.6% of the patients. 11 Urinary retention, 168 P. Cruz García-Villa et al Table 1 The International Prostate Symptom Scale (IPSS) During the last 30 days... Not at all Less than 1 in 5 times Less than half the time About half the time More than half the time Almost always 1. Over the past month, how often have you had a sensation of not emptying your bladder completely after you finish urinating? 0 1 2 3 4 5 4 5 3 4 5 2 3 4 5 2 3 4 5 3 4 5 4 times 5 times or more 2. How often have you to urinate again less than 2 hours after urinating? 0 1 2 3 3. How often have you found you stopped and started again several times when you urinate? 0 1 2 4. How often have you found it difficult to postpone urination? 0 1 5. How often have you had a weak urinary stream? 0 1 6. How often have you had to push or strain to begin urination? 0 1 2 During the last 30 days... Not at all 1 time 2 times 3 times 7. How many times did you most typically get up to urinate from the time you went to bed at night until you got up in the morning? 0 1 2 3 4 5 Total Score 0-7 Mild symptoms 8-19 Moderate symptoms 20-35 Severe symptoms incontinence, retrograde ejaculation, and erectile dysfunction are late complications.12,13 Urethral stricture is another of the late postoperative complications of TURP and is the subject of this article. The rate of urethral stricture reported in the medical literature varies from 2.2% to 9.8%.14-19 It is believed that the incidence of urethral stricture may be higher, because it depends on how and when the diagnosis is made. The World Health Organization (WHO) defines urethral stricture as a narrowing of the urethral lumen that is secondary to a scarring process that affects the erectile tissue of the corpus spongiosum causing spongiofibrosis. The contraction of the scar reduces the urethral lumen. In the instant it is sufficiently reduced to obstruct the exit flow of urine, urinary symptoms, particularly emptying ones, appear and the patient seeks medical attention. Urethral strictures or narrowings can be divided into anterior and posterior ones. Those located in the posterior urethra are invariably the consequence of trauma or radical prostatectomy. It is necessary to recall the anatomy of the urethra to adequately understand the following pathologic description. The bulbous urethra is eccentrically placed in relation to the corpus spongiosum in the bulbar portion of the urethra and is much closer to the dorsum of the penile structures. As it gets closer to Risk factors for posterior urethral stricture following TURP 169 B C A D Figure 1 Structure of the urethra. Anatomy and cross-sectional views. Different sites of the urethra are illustrated in the crosssectional views: A) at the bulbous level, B) the mid-penile level C) the distal penile level, and D) at the navicular fossa. the glans penis, the urethra is located more centrally within the corpus spongiosum (fig. 1). The corpus spongiosum receives its irrigation from the penile artery that, in turn, is a branch of the internal pudendal artery. Any situation causing the formation of a scar inside the urethra is considered to be able to produce stricture. However, the main cause of urethral stricture is trauma. Unfortunately, iatrogenic trauma can be caused during any urethral manipulation, such as the placement of a catheter or diagnostic and/or therapeutic instrumentation in treating a urinary tract pathology. Some years ago, the frequency of urethral narrowings secondary to Neisseria gonorrhoeae and Chlamydia infection was higher. Today these infections are rare, thanks to the available treatments. A very strong relation has been found principally between lichen sclerosus, or balanitis xerotica obliterans, and meatal stricture, as a consequence of the very severe inflammatory process produced. In general, patients present with obstructive and irritative urinary symptoms that are secondary to urinary infections. On occasion the patient complains of bifurcation and progressive weakening of the urinary stream that can lead to urinary retention. The introduction of a catheter can determine the presence and location of the narrowing. Before deciding on management, it is important to precisely determine the location, length, depth, and density of the stricture. This can be done through well-validated imaging studies such as urethrography and cystourethrography for establishing the location and length. Urethral ultrasound imaging identifies the density and depth of the stricture. Stricture can also be diagnosed through urethroscopy, but it is an invasive procedure and does not provide complete information about the stenosis. Once diagnosed, the stricture can be identified according to the classification established by Jordan in 1987 (fig. 2).20 The main causes of post-TURP urethral stricture are associated with location. Meatal strictures are related to a proctoscope size that is greater than the size of the urethra, whereas bulbous strictures are related to the passage of monopolar current through the sheath of the proctoscope due to an insufficient amount of lubricant. It has been proposed that the lubricant should be applied to the meatus and all along the length of the proctoscope and application should be abundant and repeated in longer procedures. Likewise, the monopolar current used should not be very high so that urethral tissue damage is prevented.13,21,22 To preserve the urethra’s normal physiology, there should be a minimum of urethral manipulation, a small caliber proctoscope should be employed, there should be adequate blood circulation, and the postoperative Foley catheter should be used for the least amount of time possible. The most frequent postoperative urethral stricture sites are the external meatus, at the level of the penoscrotal junction, the bulbous urethra, and under the sphincter.23,24 The most frequent late TURP complications are urethral stricture and sclerosus of the bladder neck, which can present in up to 9.2% of patients. Despite the technologic advances in the instrumentation, lubricants, and energy used, 170 P. Cruz García-Villa et al A D B E C F Taken from: Wein AJ, et al.6 Figure 2 Jordan classification for urethral strictures. A) Mucosal fold. B) Iris constriction. C) Full-thickness involvement with minimal spongiofibrosis. D) Full-thickness spongiofibrosis. E) Inflammation and fibrosis affecting tissues outside the corpus spongiosum. F) Complex stricture complicated by a fistula. these complication rates have not varied. Supposedly, the use of new technologies such as laser and bipolar energy reduces the risk for stricture.25,26 However, recent publications have compared the use of bipolar energy for resection with monopolar energy and reported a higher rate of urethral stricture (6.1 vs. 2.1); this was mainly attributed to having to use a wider resection sheath.27 Kuntz et al. reported similar urethral stricture rates upon comparing the holmium laser for resection and TURP, when a proctoscope was used for prostate tissue morcellation. 28,29 These results show a multifactorial risk for the development of post-TURP urethral stricture that is dependent on factors such as technique, surgery duration, antibiotic regimen used, the use of a catheter, its material, and the length of time it is indwelling, etc.11 Methods A retrospective study was conducted after receiving authorization from the Research and Ethics Committee of the Hospital Regional Lic. Adolfo López Mateos. All male patients over the age of 18 years that underwent TURP at the Urology Service of the Hospital Regional Lic. Adolfo López Mateos of the ISSSTE, and that had a preoperative diagnosis of prostatic hyperplasia, were included in the study. All patients with a past history of urethral trauma and/or pelvic fracture, a history of lithuria, patients previously treated with a modality other than TURP, not having studies confirming urethral stricture, or presenting with a previous urethral pathology were excluded. Those patients with no case records were eliminated from the study. Voiding cystourethrogram and/or cystoscopy study were reviewed to confirm urethral stricture diagnosis in those patients with suggestive symptoms. The patients were divided into 2 groups: group A: those patients with post-TURP urethral stricture diagnosis and group B: those patients that did not present with post-TURP urethral stricture diagnosis. The following variables were recorded and analyzed: age, past history of pathology, diabetes mellitus diagnosis, high blood pressure diagnosis, recurrent urinary tract infections, prostate size obtained through ultrasound, prostate-specific antigen (PSA) prior to surgery, IPSS, catheter use prior to surgery, length of time of catheter use prior to surgery, urethral dilation prior to surgery, urethral stricture at the time of surgery, type of prostatic growth, duration of resection in minutes, volume of intraoperative blood loss, volume of resected prostate tissue, caliber of postoperative transurethral catheter, material of postoperative transurethral catheter, length of time the postoperative transurethral catheter was indwelling, the length of time between TURP and stricture diagnosis, stricture location, and the method employed for stricture diagnosis. Risk factors for posterior urethral stricture following TURP 171 Table 2 Demographic characteristics of group A and group B Age Group a (n = 30) with stricture Group b (n = 33) without stricture p 64.0 ± 8.35 68.48 ± 8.76 <0.005 Diabetes mellitus 2 16.7% (5) 18.2% (6) NS High blood pressure 36.7% (11) 27.3% (9) NS Previous UTI 33.3% (10) 27.3% (9) NS Prostate volume (g) 62.93 ± 27.58 87.30 ± 60.83 <0.005 PSA (ng/mL) 6.31± 4.73 6.15 ± 3.3 NS Previous catheter 36.7% (11) 69.7% (23) <0.005 Days with catheter 38.67 ± 76.87 82.61 ± 78.97 <0.005 Pre-TURP dilation 16 (53.3%) 16 (48.5%) NS Surgery duration 57.17 ± 17.74 57.12 ± 20.04 NS 60% (18) 42.4% (14) NS Time over 60 minutes Time under 60 minutes Approximate blood loss (mL) Resected volume (g) Days with post-TURP catheter 40% (12) 57.6% (19) NS 333.33 ± 188.15 313.64 ± 136.51 NS 28.87 ± 12.42 32.0 ± 16.0 NS 8.90 ± 3.91 5.15 ± 3.0 <0.05 UTI: urinary tract infection; PSA: prostate-specific antigen; NS: not significant; TURP: transurethral resection of the prostate After the data were collected, both groups were compared. The continuous variables were compared using the Student’s t test. Means, standard deviation, frequencies, and percentages of the collected data were obtained. Thirty patients for each group were analyzed. Results In accordance with the established criteria, a total of 63 patients were included in the study; 30 belonged to the group that developed stricture (group A) and 33 to the group that did not develop stricture (group B). The demographic data are included in table 3. Of the group A patients, 33.3% (n=10) had a history of urinary tract infection prior to the TURP, whereas that figure was 27.3% for the group B patients. The prostate volume calculated by ultrasound prior to surgery was significantly different in the 2 groups, with 62.93 ± 27.58 g for group A and 87.30 ± 60.83 g for group B (p<0.005). The PSA results were very similar in the 2 groups, with 6.31± 4.73 ng/mL for group A and 6.15 ± 3.3 ng/mL for group B. The IPSS prior to the TURP for group A was 19.03 ± 3.78 points and for group B was 19.48 ± 5.42. The postoperative IPSS for group A was 16.27 ± 5.12 points and for group B was 8.88 ± 4.20 points (table 3). A Foley catheter prior to prostate surgery was used by 36.7% (n=11) of the patients that developed urethral stricture, and 69.7% (n=23) of the patients that did not develop stricture used a catheter at some point before surgery (p<0.005). The length of time that the group A patients used a catheter at some time prior to TURP was 38.67 ± 76.87 days vs. 82.61 ± 78.97 days for group B, with a p<0.005. Table 3 Pre and Post-TURP International Prostate Symptom Scale (IPSS) in group A and group B IPSS Preoperative Postoperative Group A 19.03 ± 3.78 16.27 ± 5.12 Group B 19.48 ± 5.42 8.88 ± 4.20 Urethral dilation was performed in a total of 32 patients prior to TURP; in 16 group A patients (53.3%) and in 16 group B patients (48.5%). It is important to mention that the transurethral resection equipment used on all patients of both groups had a 25.6Fr caliber sheath. In accordance with the cystoscopic findings and the modified Randall classification for the macroscopic description of the prostate gland, group A had one patient with type A, 13 patients with type B, 7 patients with type C, and 9 patients with type D. Group B had one patient with type A, 7 patients with type B, 11 patients with type C, and 14 patients with type D. Surgery duration for group A was 57.17 ± 17.74 minutes vs. 57.12 ± 20.04 minutes for group B. In group A, 60% (n=18) of the patients had a duration longer than 60 minutes and 40% (n=12) had a duration under 60 minutes, whereas in group B, 42.4% (n=14) had a duration longer than 60 minutes and 57.6% (n=19) had a duration under 60 minutes. The intraoperative blood loss for group A was 333.33 ± 188.15 mL vs. 313.64 ± 136.51 mL for group B. The quantity of resected tissue was 28.87 ± 12.42 g for group A and 32.0 ± 16.0 g for group B. 172 A 22Fr caliber catheter was used post-TURP in 28 group A and in 28 group B patients. In all patients, the postoperative catheter used was made of latex. The post-TURP Foley catheter remained indwelling for 8.90 ± 3.91 days in the group with stricture vs. 5.15 ± 3.0 days in the group without stricture (p<0.05). The mean presentation time of urethral stricture in the post-TURP patients was 40 months. Stricture location in the study group was distributed as follows: meatal stricture 3.3% (n=1), penile stricture 33.33% (n=10), bulbous stricture 73.3% (n=22), and bladder neck sclerosis 10% (n=3). It is important to mention that some patients presented with more than one stricture in different locations. Cystoscopy was carried out in 43.3% (n=13) of the patients with stricture, whereas 63.3% (n=19) underwent voiding cystourethrography. Some patients had both of the diagnostic studies done. According to the Jordan classification for urethral strictures, they were distributed as shown in figure 3. Discussion Urethral stricture frequency as reported in the medical literature varies from 2.2% to 9.8%.14-19 The World Health Organization (WHO) defines urethral stricture as a narrowing of the urethral lumen that is secondary to a scarring process, affecting the erectile tissue of the corpus spongiosum that results in spongiofibrosis. Scar contraction reduces the urethral lumen. The study population was made up of 2 groups of patients: group A were those with urethral stricture following a TURP and group B were those that underwent TURP but did not develop urethral stricture. In relation to the demographic characteristics of both groups, it should be stressed that there was a significant age difference between the 2 groups; the mean age in group A was 64.0 ± 8.35 years and it was 68.48 ± 8.76 years in group B (p<0.005). The age in the group of patients with stricture was significantly lower than that of the control group without stricture. This finding can perhaps be explained by the fact that there is better cicatrization and tissue repair after an injury or trauma in younger patients. Studies such as those by DuNuoy and Carrell found that cicatrization was better in younger patients.30 This leads to the idea that advanced age could become a protective factor for the development of urethral stricture because repair would be less intense at the site of the urethral damage, reducing the amount of fibrosis and the consequential urethral narrowing. Diabetes mellitus has been shown to substantially interfere with cicatrization processes in the entire organism. One of the contributing factors is the reduced inflammatory reaction that is associated with hyperglycemia. Diabetes diminishes granulocyte chemotaxis, phagocyte function, and cellular and humoral immunity. In addition, associated microangiopathy decreases the blood supply to the cicatrization site.31,32 The number of patients with diabetes mellitus was very similar in the 2 groups (5 and 6 in group A and group B, respectively), representing a percentage lower than 20%. Our P. Cruz García-Villa et al 3% Type A 19% 25% Type B Type C Type D Type E Type F 53% Figure 3 Stricture distribution. study results suggest that diabetes mellitus is not a risk factor for the development of urethral stricture. The same holds true for high blood pressure and a history of urinary tract infections, given that the figures did not show a tendency toward any specific group that could be interpreted as a factor intervening in the development of urethral stricture. With respect to prostate gland characteristics prior to TURP, the volumes measured by transabdominal or transrectal ultrasound showed mean values that were lower for the urethral stricture group, with 62.93 g vs. 87.3 g for the group that did not develop stricture and a p<0.05. Those patients with higher prostate volumes had a lesser tendency to develop urethral stricture. This is quite striking, given that a higher prostate volume implies a longer resection time. This result could be attributed to the fact that prostate volume measurement was indistinctly carried out, either transabdominally or transrectally, resulting in volume variability, depending on the method employed. Further studies could corroborate whether these same findings are present in larger populations. In relation to the high prostate volumes, it is not surprising that the PSA figures were above normal values in the 2 groups, and there was no difference between them. In both groups the IPSS scale showed a decrease after TURP. The mean initial IPSS in the 2 groups was found to be in the moderate symptom range, with 19 points for each group. After TURP, the group without stricture showed a descent of 10 points on the scale vs. a descent of 3 points in the patients that developed urethral stricture. It was not possible to precisely know the postoperative IPSS at a determined point in time due to the fact that the measurements were taken in the patients at different post-TURP moments. Nevertheless, it is clear that the patients that presented with stricture developed symptoms with greater frequency and intensity than those that did not present with stricture after TURP. There were patients that had indwelling transurethral catheters as temporary treatment at some point prior to TURP in the 2 groups. Of those patients that did not develop Risk factors for posterior urethral stricture following TURP urethral stricture, 69.7% (n=23) had used a catheter at some moment vs. 36.7% (n=11) of those patients that developed stricture (p<0.05). Likewise, those that did not develop stricture and that used a catheter prior to TURP had a mean indwelling time of 82.6 days vs. 36.7 days in those patients that developed stricture. This suggests that the prolonged use of a transurethral catheter before TURP creates a urethral inflammation episode that protects the patient or is conducive to making the second inflammatory episode from the placement of a catheter after TURP less intense and shorter. In other words, the inflammatory process is not so severe in those patients that have had previous contact with the material of the catheter (latex, in the majority of the cases), thus reducing the possibility of developing urethral stricture. As was to be expected, of those patients that developed urethral stricture, 53% had received dilation prior to TURP vs. 48% of the patients that did not develop stricture. Urethral dilation is undeniably a risk factor for urethral trauma with injury to the mucosa that can condition the formation of spongiofibrosis and urethral stricture. Therefore, gentle dilation is recommended, using the adequate amount of lubricant so as not to injure the urethra at any point along its course. The anatomic configuration of the prostate according to the modified Randall classification showed that type B (n=11) was the most frequent in the patients that developed stricture (group A) followed by types D (n=9) and C (n=7). In group B the most frequent type was the Randall D (n=14), followed by types B (n=11) and C (n=7). The mean surgery duration for both groups was 57.17 ± 17.74 minutes for group A and 57.12 ± 20.04 minutes for group B. However, as established in the hypothesis, 60% (n=18) of the patients that developed stricture (group A) had a resection time above 60 minutes vs. 42.4% (n=14) of the patients that did not develop stricture (group B). According to reports in the medical literature, resection time is one of the most important factors for developing urethral stricture.33,34 The results of our study showed a coinciding tendency for a TURP duration greater than 60 minutes to be a risk factor for the later development of urethral stricture, although there was no statistically significant difference. The intraoperative blood loss for both groups was very similar, with a mean 333 mL for group A and 313 mL for group B. The quantity of resected tissue was also similar in the 2 groups, with a mean 28 g for group A and 32 g for group B. In this aspect, it is worth mentioning that time, blood loss, and resected volume were correlated, taking into account that resection time was intended to be no greater than 60 minutes, and blood loss was calculated based on the quantity of resected tissue, multiplying the resected volume by 10 mL. After TURP, a difference in the indwelling time of the transurethral catheter was observed between the 2 groups. Those patients that had the indwelling catheter for a longer period of time after the TURP had a higher percentage of probability of developing stricture. In group A the mean length of time with catheter after TURP was 8.90 ± 3.91 days and in group B it was 5.15 ± 3.0 days with a p<0.05. This is due to the fact that the postoperative inflammatory process disappears within the first 48 to 72 hours. The inflammatory process in those patients that have a catheter 173 for more time is more intense and prolonged as a consequence of the presence of a foreign body at the surgical site and along the course of the urethra. A descriptive analysis was done on the Group A patients. In those patients the length of time from the TURP to the appearance of stricture was a mean 40.7 months (range: 4 to 70 months). In regard to stricture location the distribution was as follows: bulbous urethra 73% (n=22), penile urethra 33.3% (n=10), bladder neck 10% (n=3), and meatus 3.3% (n=1). Two strictures in 2 different locations were found in 6 patients. These results are in contrast to those of another study in which stricture incidence was greater in the meatus (18.3%) than in the bulbous urethra (9.1%).1 This difference could be due to the fact that the aim of that study was to show a decrease in stricture using an irrigation solution at a temperature of 36° C, which reduced the incidence of bulbous and penile strictures, but not meatal strictures. Taking into account the Jordan classification described in 1987 based on spongiofibrosis configuration and extension, the strictures were divided into type B 56.7% (n=17), type C 26.7% (n=8), type A 20% (n=6), and type D 3.3% (n=1).20 There was no type E stricture, albeit that this type was difficult to determine, given that none of these patients had a urethral ultrasound study to establish the extension of the fibrosis into the corpora cavernosa (type E stricture). There was no type F stricture (associated with fistula). Of the 30 patients, 19 had voiding cystourethrography, whereas 13 had cystoscopy in order to diagnose urethral stricture. Both studies were carried out on 2 patients due to inconclusive voiding cystourethrography. Urethral ultrasound was not done on any of these patients because it is not a routine study in our service. Urethral ultrasound imaging is an important study because it determines stricture depth, enabling appropriate classification, which in turn results in more adequate treatment for these patients. Conclusions Based on the results of our study, the main risk factors for the formation of urethral stricture in patients that have undergone TURP are a preoperative prostate volume greater than 80 g that is determined through transabdominal or transrectal ultrasound imaging, dilation of the urethra immediately prior to the procedure, a resection time greater than 60 minutes, and the prolonged use (8.9 ± 3.91 days) of a postoperative transurethral catheter. According to the medical literature, there may be other additional factors in the development of urethral narrowing, such as an insufficient quantity of intraurethral lubricant before and after the surgery, the use of a high level of energy for cutting and coagulating that causes the sheath to heat up during the procedure, the use of a sheath with a diameter greater than that of the urethra, the material from which the catheter is made, and the temperature of the irrigation solution during the procedure. All these factors should continue to be analyzed, because each one of them can have an effect on the process of epithelial damage and the local inflammation that are later produced, with the probability of causing scarring that results in a urethral stricture. 174 Conflict of interest The authors declare that there is no conflict of interest. Financial disclosure No financial support was received in relation to this article. References 1. Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol 2005;7 Suppl 9:S3-S14. 2. Bierhoff E, Vogel J, Benz M, et al. Stromal nodules in benign prostatic hyperplasia. Eur Urol 1996;29(3):345-354. 3. Meigs JB, Mohr B, Barry MJ, et al. Risk factors for clinical benign prostatic enlargement in a community-based population of healthy aging men. J Clin Epidemiol 2001;54(9):935-944. 4. Michel MC, Mehlburger L, Schumacher H, et al. Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. J Urol 2000;163(6):1725-1729. 5. Verhamme K, Dieleman J, Bleumink G, et al. Incidence and prevalence of lower urinary tract symptoms suggestive of benign prostatic enlargement in primary care-the Triumph project. Eur Urol 2002;42(4):323-328. 6. Wein AJ, Kavoussi RL, Novick AC, et al. Campbell-Walsh. Urología. 9a edición. Tomo 3, capítulo 86. Buenos Aires: Editorial Panamericana; 2007. p. 2740. 7. Thorner DA, Weiss JP. Benign Prostatic Hyperplasia: Symptoms, Symptom Scores, and Outcome Measures. Urol Clin North Am 2009;36(4):417-429. 8. Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol 1992;148(5):1549-1557. 9. Clasificación de Randall original y modificada. Surgical pathology of Prostatic Obstruction. Baltimore: Williams and Wilkins; 1931. p. 2735. 10. Wasserman N. Hiperplasia benigna de la próstata: revisión y clasificación ecográfica. Radiol Clin North Am 2006;44(5):689710. 11. Colau A, Lucet JC, Rufat P, et al. Incidence and risk factors of bacteruria after transurethral resection of the prostate. Eur Urol 2001;39(3):272-276. 12. Madersbacher S, Marberger M. Is transurethral resection of the prostate still justified? BJU Int 1999;83(3):227-237. 13. Hoffmann R. Transurethrale Resektion (TURP) und transurethrale Inzision (TUIP) der Prostata. In: Hoffmann R (editor). Endoskopische Urologie. Heidelberg: Springer; 2005. p. 50-84. 14. Kwan Park J, Kyi Lee S, Hee Han S, et al. Is Warm Temperature Necessary to Prevent Urethral Stricture in Combined Transurethral Resection and Vaporization of Prostate? Urology 2009;74(1):125-129. 15. Souverein PC, Erkens JA, de la Rosette JJ, et al. Drug treatment of benign prostatic hyperplasia and hospital admission for BPHrelated surgery. Eur Urol 2003;43(5):528-534. 16. Mebust WK, Holtgrewe HL, Cockett AT, et al. Transurethral prostatectomy: immediate and postoperative complications—a P. Cruz García-Villa et al cooperative study of 13 participating institutions evaluating 3885 patients. J Urol 1989;141(2):243-247. 17. Tan A, Liao C, Mo Z, et al. Meta-analysis of holmium laser enucleation versus transurethral resection of the prostate for symptomatic prostatic obstruction. Br J Surg. 2007;94(10):12011208. 18. Hammadeh MY, Madaan S, Hines J, et al. 5-Year outcome of a prospective randomized trial to compare transurethral electrovaporization of the prostate and standard transurethral resection. Urology 2003;61(6):1166-1171. 19. Rassweiler J, Teber D, Kuntz R, et al. Complications of transurethral resection of the prostate (TURP)—incidence, management, and prevention. Eur Urol 2006;50(5):969-979. 20. Jordan GH. Management of anterior urethral stricture disease. Clin Plast Surg 1988;15(3):493-505. 21. Hartung R, May F. Die transurethrale Elektroresektion der Prostata. BJU Int 2006;98(4):921-934. 22. Faul P. Video-TUR: raising the gold standard. Eur Urol 1993;24(2):256-261. 23. Blandy HP, Notley RG, Reynard JM. Transurethral Resection, 5th ed. London: Taylor & Francis; 2005. p. 183-196. 24. Aron M, Goel R, Gautam G, et al. Percutaneous versus transurethral cystolithotripsy and TURP for large prostates and large vesical calculi: refinement of technique and updated data. Int Urol Nephrol 2007;39(1):173-177. 25. Wendt-Nordahl G, Häcker A, Reich O, et al. The Vista system: a new bipolar resection device for endourological procedures: comparison with conventional resectoscope. Eur Urol 2004;46(5):586-590. 26. Rassweiler J, Frede T, Seemann O, et al. Medium power Ho: YAG lasers. In: Gupta NP, Kumar R, editors. Holmium laser – Endourological applications. New Dehli: B.I. Publications; 2003. p. 58–61. 27. Tefekli A, Muslumanoglu AY, Baykai M, et al. A hybrid technique using bipolar energy in transurethral prostate surgery: a prospective, randomized comparison. J Urol 2005;174(4 Pt 1):1339-1343. 28. Kuntz RM, Ahyai S, Lehrich K, et al. Transurethral holmium laser enucleation of the prostate versus transurethral electrocautery resection of the prostate: A randomized prospective trial in 200 patients. J Urol 2004;172(3):1012-1016. 29. Starkman JS, Santucci RA. Comparison of bipolar resection of the prostate with standard transurethral prostatectomy: shorter stay, earlier catheter removal and fewer complications. BJU Int 2005;95(1):69-71. 30. DuNuoy P, Carrell A. Cicatrization of wounds. J Exp Biol 1921;34:339. 31. BybeeJD, Rogers DE. The phagocytic activity of polymorphonuclear leukocytes obtained from patients with diabetes mellitus. J Lab Clin Med 1964;64:1-13. 32. Yue DK, McLennan S, Marsh M, et al. Effects of experimental diabetes, uremia, and malnutrition on wound healing. Diabetes 1987;36(3):295-299. 33. Blandy HP, Notley RG, Reynard JM. Transurethral Resection, 5th ed. London: Taylor & Francis; 2005. p. 183-196. 34. Aron M, Goel R, Gautam G, et al. Percutaneous and transurethral cystolithotripsy versus TURP for large prostates and large vesical calculi: refinement of technique and updated data. Int Urol Nephrol 2007;39(1):173-177. Rev Mex Urol 2013;73(4):175-179 ÓRGANO OFICIAL DE DIFUSIÓN DE LA SOCIEDAD MEXICANA DE UROLOGÍA www.elsevier.es/uromx Original article Oncologic effectiveness and safety of laparoscopic renal cryosurgery guided by high definition laparoscopic ultrasound J. G. Campos-Salcedoa,*, G. Hernández-Martínezb, E. I. Bravo Castrob, A. SedanoLozanoc, J. C. López-Silvestred, M. Á. Zapata-Villalbae, L. A. Mendoza-Álvarezf, C. E. Estrada-Carrascof, H. Rosas-Hernándezf, C. Díaz-Gómezf, C. Paredes-Calvaf y J. L. ReyesEquihuaf a Urology Service Administration, Hospital Central Militar, Mexico City, Mexico b Urology Speciality Residency, Escuela Militar de Graduados de Sanidad, Mexico City, Mexico c Clinical Administration of Medical Specialities, SEDENA, Mexico City, Mexico d Urology Ward Administration, Hospital Central Militar, Mexico City, Mexico e Urology Operating Room Administration, Hospital Central Militar, Mexico City, Mexico f Urology Service, Hospital Central Militar, Mexico City, Mexico Abstract KEYWORDS Cryoablation; Renal cryosurgery; Renal tumor; Cryocatheter; Laparoscopic cryotherapy; Mexico. Background: The necessity and desire for definitive treatment in T1 tumors in patients that had previously been considered inoperable has resulted in the addition of cryoablation to the treatment armamentarium. Aims: To determine the experience, results, and complications of this treatment in our hospital center. Material and methods: Laparoscopic renal cryoablation guided by laparoscopic ultrasound was surgically indicated in 8 renal tumor patients with multiple comorbidities at the Hospital Central Militar. Results: The mean age of the patients was 54.3 years and the mean size of the lesions was 28 mm. The lesion reduction percentage average was 47%. There were no complications of conversion, urinary fistulas, or renal loss. The incidence of clear cell carcinoma was 75%, and angiomyolipoma was present in 25% of the lesions. Discussion: The oncologic effectiveness of this management is still being defined; our results suggest that it offers a feasible, safe, and effective treatment opportunity to those patients in need of maximum nerve-sparing management. Conclusions: After a decade of international experience, there have been few studies carried out on the Mexican population. Given the favorable results of our study, we feel it is necessary to continue and promote long-term studies, and we stress the importance that learning to perform this modality has for today’s urologist. * Corresponding author at: Hospital Central Militar. Blvd. Manuel Ávila Camacho s/n, Lomas de Sotelo, Av. Industria Militar y General Cabral, Delegación Miguel Hidalgo, C.P. 11200, México D.F., México. Telephone: (01) 5557 3100, ext. 1246. Email: drjgaducampos@hotmail.com (J. G. Campos-Salcedo). 0185-4542 - see front matter © 2013. Revista Mexicana de Urología. Publicado por Elsevier México. Todos los derechos reservados. 176 Palabras clave Crioablación; Criocirugía renal; Tumor renal; Criosonda; Crioterapia laparoscópica; México. J. G. Campos-Salcedo et al Eficacia y seguridad oncológica de la criocirugía renal laparoscópica guiada con ultrasonido laparoscópico de alta definición Resumen Introducción: La necesidad y el deseo de tratamiento definitivo en tumores T1a en pacientes que antes habrían quedado fuera de tratamiento quirúrgico, ha lanzado a la crioablación como una herramienta más en su tratamiento. Objetivo: Determinar la experiencia, resultados y complicaciones de nuestro centro hospitalario. Material y métodos: Se realizó crioablación renal laparoscópica guiada por ultrasonido laparoscópico, a 8 tumores renales con indicación quirúrgica y múltiples comorbilidades, en el Hospital Central Militar. Resultados: La edad promedio de los pacientes fue 54.3 años. Las lesiones promedio de 28 mm, porcentaje promedio de reducción de las lesiones de 47%; las complicaciones como conversión, fístulas urinarias y pérdida renal del 0%, con incidencia de carcinoma de células claras en 75% y angiomiolipoma en 25% de las lesiones. Discusión: La eficacia oncológica sigue en definición. Nuestros resultados sugieren que ofrece tratamiento factible, seguro y eficaz en pacientes que requieren un máximo esfuerzo preservador de nefronas, representando una oportunidad de tratamiento. Conclusiones: A una década de experiencia en el mundo, en México se cuentan con escasos estudios en la población mexicana. Presentamos estos resultados concluyendo que es necesario continuar e impulsar estudios a largo plazo dados los resultados favorables, y por lo tanto hacemos hincapié en la importancia de su aprendizaje para el urólogo actual. Introduction Methods Twenty years ago, Dr. Andy Novick was one of the pioneers of the concept of open partial renal surgery, in an effort to promote the nephron-sparing approach as part of the oncologic principles.1 Thanks to great technological advances, both the oncologic principle and the maximum renal function are now able to be preserved. Today, the situation is as Dr. Novick had imagined it, but with the advantage that these technologies are available in most parts of the world for all patients, offering an excellent treatment alternative to the patient with multiple comorbidities. New improvements are added daily to this renal preservation that are minimally invasive and focus largely on treatment. This is the case with laparoscopy, cryosurgery, and high definition laparoscopic ultrasound, and the common objective is our patients’ wellbeing. Cryotherapy has a lethal local effect resulting from 2 sequential synergic mechanisms. The first is the so-called direct cytotoxic lesion due to the formation of ice crystal during the freezing phase, and is followed by the damage from indirect ischemia due to the occlusion of the local microvasculature during the consequent thawing phase.2 In laparoscopic renal cryoablation, the cryocatheter can be precisely positioned and the entire surgical event of ice ball formation can be monitored in real time and under direct vision through ultrasound guidance.3 The growing enthusiasm surrounding minimally invasive surgery and the need and desire for definitive treatment of T1a incidental renal tumors has turned laparoscopic renal cryoablation into another treatment alternative for small renal tumors in patients who in the past would not have been candidates for surgical treatment.4-6 A descriptive study was conducted on 8 selected patients presenting with T1 aN0M0 renal masses indicated for nephron-sparing surgery for a variety of reasons that included having only one kidney due to previous renal tumor, having kidney failure, etc. The inclusion criteria are grouped together in table 1. The exclusion criterion was if the patient did not comply with the follow-up measures dictated by our hospital center. All the patients underwent percutaneous renal biopsy, as recommended in the urologic clinical guidelines of the European Association of Urology for ablative therapies in the same surgical procedure as the renal cryoablation.5 Two cores per renal lesion were taken (fig.1). Laparoscopic renal cryoablation guided by high definition laparoscopic ultrasound was performed on 8 lesions characterized by tomography in patients with T1aN0M0 renal tumors. All the patients presented with multiple comorbidities that did not form part of the inclusion, non-inclusion, or exclusion criteria; they will be characterized further on in the text. In this case series there was no control group. At the Hospital Central Militar, with the Cryocare Surgical System (Endocare Inc., Irvine, Calif, USA) equipment, 17Ga cryocatheters were used (figs. 2 and 3) that underwent two 10-min freezing cycles. The real time formation of the ice ball was observed with 10 mHz BK Pro-Focus 2202 high definition laparoscopic ultrasonographic guide (fig. 4) until it completely covered the tumor mass and surrounded it by an approximate 8 mm margin (fig. 5). Laparoscopic renal cryosurgery 177 Tabla 1 Inclusion criteria. The patient and renal lesion inclusion criteria for undergoing laparoscopic renal cryoablation are presented. Inclusion criteria for nephron-sparing treatment through laparoscopic renal cryoablation Patient factors Unspecified sex Unspecified comorbidities Indication for nephron-sparing surgery according to the 2010 EAU Clinical Guidelines for renal cancer treatment Lesion characteristics Unilateral or bilateral renal lesion whose greatest diameter is under 4 cm Cortical renal lesion Nonspecific metastatic disease Tomographic enhancement of more than 20 HU EAU: European Association of Urology; HU: Hounsfield units. Results The mean age of the patients analyzed was 54.3 years; 87% (n=7) of the patients were operated on with the laparoscopic approach (fig. 6). Their lesions measured a mean 28 mm (40-22 mm). Only the first patient was operated on with the open technique; we decided to include this patient in our case series for the purpose of showing oncologic control results. The sequential sizes of the lesions were reduced in one case down to 0 mm, with an average lesion reduction percentage of 47%. There were no complications of conversion to open surgery, urinary fistulas, renal loss, or the need for dialysis. The mean preoperative creatinine value was 1 mg/dL and the post-cryosurgery value was 1.2 mg/dL. The histopathologic report of the biopsies stated clear cell carcinoma in 75% of the lesions and angiomyolipoma in 25%. These results are shown in table 2. It should be mentioned that one of the patients died due to causes other than the renal tumor and so the oncologic Figure 1 Biopsy guided by laparoscopy and high definition laparoscopic ultrasonography showing the Bard 15 Ga biopsy forceps at the moment of puncture. control could not be carried out. This patient was included only for the immediate postoperative progression and was eliminated in the tomographic control. Discussion The oncologic effectiveness of laparoscopic renal cryosurgery has not yet been completely defined due to the followup time in different case series, which has also been documented in the cryosurgery tendencies in Mexico. However, our results suggest that it offers a feasible, safe, and effective treatment for renal masses in patients that require maximum nephron-sparing management, providing them with an opportunity for treatment.7-10 Conclusions After more than 10 years of experience worldwide, there are only a few long and medium-term studies on the results Figure 2 The Cryocare Surgical System (Endocare Inc., Irvine, Calif, USA) 17Ga cryocatheter during renal cryoablation. Figure 3 The Cryocare Surgical System (Endocare Inc., Irvine, Calif, USA) 17Ga cryocatheter. The red arrows signal the depth of the cryocatheter needle, which is correlated with the limit of the ice ball formation (green arrows). 178 J. G. Campos-Salcedo et al Figure 5 The high definition laparoscopic ultrasound showing the ecographically homogeneous renal parenchyma with no lesion; the ice ball formation is seen as a hypoechoic area in the center of the renal parenchyma. Figure 4 The high definition laparoscopic ultrasound in contact with the renal parenchyma for cryoablation guide and ice ball formation. of this technique in the Mexican population, despite the numerous benefits it has shown. We have presented the results of a medium-term follow-up at a Mexican tertiary care hospital where laparoscopic renal cryoablation guided by high definition laparoscopic ultrasound is carried out. It is necessary to continue and promote long-term studies, given that the results are becoming more and more favorable for patients. We corroborate the fact that the application of this ablation therapy provides the patient with an opportunity for treatment, and many improvement prospects. The patient is benefitted physically through a better quality of life that is a characteristic of minimally invasive surgery, as well Figure 6 Cryoablation. The ice ball created by 2 cryocatheters in the first cooling phase and formed over the renal tumor lesion is shown. as psychologically, given that the preoccupation caused by being “surgically inoperable” is reduced, maintaining the oncologic principles. For these reasons we stress the importance and great usefulness that learning this technique has for the practicing urologist, as well as for those in training. For the benefit of the patients, it should always be kept in mind when making therapeutic decisions. Conflict of interest The authors declare that there is no conflict of interest. Table 2 Result compilation. Specific results for each lesion. The first column to the left shows the lesion number and the second column shows the TNM staging according to the 2010 European Association of Urology guidelines. Age (years) TNM Tumor size (mm) Comorbidities Pre-op creat DHS Postop creat Post-op UO ml/ kg/hr Cryolesion reduc (%) HPS 1 31 T1aN0M0 39 Right single kidney due to left Wünderlich syndrome 0.85 8 1.0 1.6 89.7 AML 2 31 T1aN0M0 42 Right single kidney due to left Wünderlich syndrome 0.85 8 1.0 1.6 89.7 AML 3 38 T1aN0M0 20 DM 0.8 8 1.0 1.4 33 ccRCC 4 72 T1aN0M0 31 Sub. Mesenteric hepatic thrombosis/DM2/HBP/ chronic liver disease 0.8 21 1.1 1.0 100% ccRCC 5 75 T1aN0M0 30 ccRCC/PN 2004 and cryo 2007/CKF/HPO/HBP 1.8 11 2.6 0.1 89% ccRCC 6 62 T1aN0M0 23 ccRCC 2007 1.1 4 1.7 0.5 15% ccRCC 7 49 T1aN0M0 20 DM2 1.0 1 0.8 1.0 15% ccRCC 8 79 T1aN0M0 22 DM2 0.8 3 1.2 1.0 0% ccRCC DM2: diabetes mellitus type 2; HBP: high blood pressure; Pre-op creat: preoperative serum creatinine; DHS: Days of hospital stay; Postop creat: Postoperative serum creatinine at 24 hours; UO: Urinary output; Cryolesion reduc: Cryolesion reduction percentage; HPS: Histopathologic study report; AML: angiomyolipoma; ccRCC: Clear cell renal cell carcinoma. Laparoscopic renal cryosurgery Financial disclosure No financial support was received in relation to this article. References 1. Gill IS, Remer EM, Hasan WA, et al. Renal Cryoablation. Outcome at 3 years. J Urol 2005;173(6):1903-1907. 2. Campbell SC, Palese MA. Opposing views. Laparoscopic cryoablation for a 3 cm nonhiliar renal tumor. J Urol 2011;185(1):1416. 3. Autorino R, Haber GP, White MA, et al. New developments in focal therapy. J Endourol 2010;24(5):665-672. 4. Gill IS, Aron M, Gervais DA, et al. Clinical practice. Small renal mass. N Engl J Med 2010;362(7):624-634. 179 5. Ljungberg B, Cowan N, Hanbury DC, et al. Guidelines on Renal Cell Carcinoma. Eur Urol 2010;58(3):398-406. 6. Davol PE, Fulmre BR, Rustalis DB. Long term results of cryoablation for renal cancer and complex renal masses. Urology 2006;68(1 Suppl):2-6. 7. Remer EM, Hale JC, Inderbir G, et al Technical Innovation. Sonographic Guidance of Laparoscopic renal cryoablation. AJR Am J Roentgenol 2000;174(6):1595-1596. 8. Heuer R, Gill IS, Guazzoni G, et al. A critical analysis of the actual role of minimally invasive surgery and active surveillance for kidney cancer. Eur Urol 2010;57(2):223-232. 9. Springer C, Hoda MR, Fajkovic H, et al. Laparoscopic vs open partial nephrectomy for T1 renal tumours: evaluation of longterm oncological and functional outcomes in 340 patients. BJU Int 2013;111(2):281-288. 10. Long CJ, Canter DJ, Smaldone MC, et al. Role of tumor location in selecting patients for percutaneous versus surgical cryoablation of renal masses. Can J Urol 2012;19(5):6417-6422. Rev Mex Urol 2013;73(4):180-186 ÓRGANO OFICIAL DE DIFUSIÓN DE LA SOCIEDAD MEXICANA DE UROLOGÍA www.elsevier.es/uromx Original article Usefulness of urethral ultrasound imaging in urethral stricture P. Cruz García-Villaa,*, M. Figueroa-Zarzab, D. López-Alvaradoa and F. Mendoza-Peñac a Urology Speciality Residency, Hospital Regional “Lic. Adolfo López Mateos”, ISSSTE, Mexico City, Mexico b Department of Urology, Hospital Regional “Lic. Adolfo López Mateos”, ISSSTE, Mexico City, Mexico c Department of Urology Administration, Hospital Regional “Lic. Adolfo López Mateos”, ISSSTE, Mexico City, Mexico KEYWORDS Stricture; Urethra; Ultrasound; Mexico. Abstract Background: Urethral stricture is defined as a narrowing secondary to tissue scarring. Diagnosis is made with contrast-enhanced imaging studies such as cystourethrography. Urethral ultrasound is a noninvasive imaging method that enables the diagnosis and classification of urethral stricture. Material and methods: Thirty patients with a past history of urethral stricture underwent urethral ultrasound. The strictures were measured, a questionnaire on urethral ultrasound was applied, and a descriptive data analysis was done. Results: The mean age of the patients was 66 years. A total of 33.3% patients underwent cystoscopy and 73% had cystourethrography. In 50% of the patients, initial treatment was urethral dilation. Significant urethral stricture was found through ultrasound in 80% of the patients. The mean stricture length was 0.84 cm and the mean depth was 0.37 cm. The patients experienced less “discomfort” during the ultrasound procedure and would recommend it over voiding cystourethrography (VCUG) and/or cystoscopy. Discussion: Urethral ultrasound is a noninvasive imaging method that identifies stricture location and length and evaluates the depth of the spongiofibrosis. Cystourethrography can underestimate stricture length and it does not provide information on depth and density. Ultrasound imaging should be complementary in patients with urethral stricture and should be used for surgical planning and adequate follow-up. * Corresponding author at: Av. Universidad N° 1321, Colonia Florida, Delegación Álvaro Obregón, C.P. 01030, México D.F., México. Telephone: 5322 2300. Email: patricio_cruzgar@yahoo.com.mx (P. Cruz García-Villa). 0185-4542 - see front matter © 2013. Revista Mexicana de Urología. Publicado por Elsevier México. Todos los derechos reservados. Urethral ultrasound Palabras clave Estenosis; Uretra; Ultrasonido; México. 181 Utilidad ultrasonido uretral en estenosis de uretra Resumen Introducción: La estenosis de uretra se define como una estrechez secundaria a tejido cicatrizal. El diagnóstico se realiza con estudios de imagen con medio de contraste, tal como la uretrocistografía. El ultrasonido uretral es un método de imagen no invasivo, que permite diagnosticar y clasificar la estenosis de uretra. Material y métodos: Se realizó ultrasonido uretral en 30 pacientes con antecedente de estenosis de uretra. Se midieron las estenosis. Se realizó un cuestionario sobre el ultrasonido uretral. Se hizo un análisis descriptivo de los datos. Resultados: La edad promedio fue de 66 años. El 33.3% tenía cistoscopia y el 73% uretrocistografía. En el 50% el tratamiento inicial fue con dilatación. En el 80% de los pacientes se encontró estenosis de uretra significativa por ultrasonido. La longitud promedio fue de 0.84 cm y la profundidad promedio fue de 0.37 cm. Los pacientes sintieron menos “molestia” durante el ultrasonido, y lo recomendarían más que la uretrocistografía miccional (UCGM) y/o la cistoscopia. Discusión: El ultrasonido uretral es no invasivo. Permite obtener la localización y la longitud, así como la valoración de la profundidad de la espongiofibrosis. La uretrocistografía puede subestimar la longitud, y no informa la profundidad y densidad. El ultrasonido debe ser complementario en los pacientes con estenosis de uretra. El ultrasonido debe hacerse para una planeación quirúrgica y un adecuado seguimiento. Introduction Methods According to information from the U.S. Veterans Affairs hospital database the rate of urethral stricture was 193/100,000 for the year 2003. According to this database, the stricture rate increased significantly in patients above the age of 55 years. However, the incidence of urethral stricture is unknown. Medical consultations for urethral stricture determined by the U.S. National Ambulatory Medical Care Survey within the time frame of 1992 to 2000 were reported at a rate of 229/100,000.1 Urethral stricture is defined as a narrowing of the urethral lumen secondary to scar tissue. Etiology can be infectious, iatrogenic, traumatic, or idiopathic. These patients present with urinary symptomatology that affects quality of life and they are often offered procedures such as dilation or optical internal urethrotomy (OIU). In some cases the patients may develop severe symptoms such as kidney failure, acute urine retention, urethral carcinoma, Fournier’s gangrene, and bladder dysfunction as a consequence of the stricture.2 According to the same survey, the number of retrograde urethrograms carried out on the population over 65 years of age was 6,557/100,000 in 2001. In other words, 6.5% of the patients above the age of 65 years with urethral stricture underwent that study.1 Numerous studies have shown that urethral ultrasound imaging offers greater precision in determining stricture length.3,4 Ultrasound as a diagnostic tool for urethral stricture offers the advantages of being a non-invasive study that enables the anterior urethral strictures to be seen rapidly, simply, and precisely. Likewise, it measures stricture length and depth more exactly than retrograde urethrography. A descriptive study was conducted on 30 men using urethral ultrasound as a support tool in the management of patients presenting with urethral stricture. The study was carried out at the Urology Service of the Hospital Regional Lic. Adolfo López Mateos of the ISSSTE. Prior to their participation, all patients signed informed consent statements. The ultrasound equipment used was the Esaote Mylab™ Desk with a 7.5 mHz linear transducer. With the patient in the dorsal decubitus position, a 12Fr Foley catheter was placed in the navicular fossa and with a continuous drip through the catheter, an ultrasound sweep of the anterior urethra was carried out. All the studies were performed by the same physician. The location, length, and depth of the strictures found were recorded. After the ultrasound, a still non-validated questionnaire that was created in stages was applied to identify the grade of discomfort of the ultrasound study, to be compared with cystoscopy and retrograde urethrocystography. A descriptive analysis of the data was done, obtaining means, standard deviation, and frequencies. Results An ultrasound study of the urethra was carried out on 30 male patients. Their mean age was 66 ± 9.1 years. A total of 30% of the patients had a past history of diabetes mellitus type 2, 46.7% high blood pressure, 13.3% heart disease, and 10% had a history of kidney failure. Ten percent of the patients had prostate cancer and 13.3% presented with some other comorbidity. A total of 24 patients (80%) had a past history of TURP and 21 (70%) had been treated with OIU. A total of 13.3% of the patients had undergone open or radical prostatectomy and 23.3% had some other surgery. 182 P. Cruz García-Villa et al Figure 1 A) and B) Voiding cystourethrogram that shows stricture data at the penoscrotal junction and the bulbous urethra. C) and D) Urethral ultrasound that shows a reduction in the caliber of the urethra at the level of the penoscrotal junction and the bulbous urethra. Spongiofibrosis surrounding the tissue can be seen. Of the patients with previous urologic surgery, the mean time of stricture diagnosis after the surgery was 26.3 ± 24.5 months. Thirty-three percent of the patients had a cystoscopic study, whereas 73.3% had cystourethrography. Of the patients that had cystoscopy, 80% presented with urethral stricture. Ninety-five percent of the patients that had cystourethrography presented with urethral stricture data (fig. 1). Of the patients that had some kind of diagnostic study (cystoscopy and/or cystourethrography), 70% presented with stricture in the bulbous urethra, 20% in the penile urethra, and 13% in the prostatic urethra. Initial treatment was dilation or calibration in 46.7% of the patients. OIU was done on 23.3%. Treatment was transurethral catheter placement or cystostomy in 16.7% of the patients, and there was no initial treatment in 13% (table 1). Prior to the ultrasound, the International Prostate Symptom Score (IPSS) questionnaire was applied to all the patients. Forty percent of them had a moderate score (8 to 19 points), 30% had a severe score (20 to 35 points), and 30% had a mild score (1 to 7 points). Urethral stricture was found in 80% (n=24) of the patients that had ultrasound; it was situated in the penile urethra in 36.7%, in the bulbous urethra in 40%, and in the membranous urethra in 3.3%. A second stricture was found in the bulbous urethra in 6 cases and a third stricture was found in the bulbous urethra in 2 cases. In 89.3% of the patients, the location of the stricture found through ultrasound imaging coincided with the location found through cystoscopy and/or cystourethrography. The mean number of strictures found was 1 ± 0.78, the mean stricture length was 0.84 ± 0.50 cm, and the mean depth was 0.37 ± 0.17 cm (table 2). In relation to the responses to the questionnaire applied after urethral ultrasonography, 79.3% of the patients said there was less discomfort with the ultrasound study than with the previous study (cystoscopy and/or cystourethrography), 17.2% stated there was more discomfort, and 3.4% said the discomfort was the same. The ultrasound study was regarded as less invasive by 75.9% of the patients and 86.2% considered that it took less time than the previous study. A total of 72.4% stated that they had felt pain with the cystoscopy and/or cystourethrography. Of those patients, the mean pain score was 5 ± 3.36 points, according to the visual pain analog scale. On the other hand, 48.3% of the patients stated they had felt pain with the ultrasound study and had a mean pain score of 2.2 ± 2.7 points. Urethral ultrasound 183 Table 1 General patient characteristics Age (years) 66 ± 9.1 N=30 Table 2 Urethral stricture location percentage and length and depth through ultrasonography Patients with urethral ultrasound Finding of stricture DM2 30% HBP 46.7% Penile urethra Heart disease 13.3% Bulbous urethra N=30 80% (24) 36.7% 40% CKF 10% Membranous urethra Prostate cancer 10% 89.3% (25) Previous TURP 80% Coinciding with cystoscopy and/or cystourethroscopy Previous OIU 70% Length (cm) 0.84 ± 0.50 Depth (cm) 0.37 ± 0.1 Cystoscopy 33.3% Cystourethrography 73.3% 3.3% Initial treatment Dilation 46.7% OIU 23.3% Others 16.7% Without treatment 13.3% DM2: diabetes mellitus type 2; HBP: high blood pressure; CKF: chronic kidney failure; TURP: transurethral resection of the prostate; OIU: optical internal urethrotomy. Thirty-one percent of the patients that had cystoscopy and/or cystourethrography reported having had some kind of complication such as hematuria, pain, micturition difficulty, infection, etc. A total of 55.2% of this group of patients presented with dysuria at some point after the procedure. Ninety-six percent of the patients said they had none of the abovementioned complications after urethral ultrasonography and 6.7% of the patients in this group presented with dysuria after the ultrasound study. The greatest discomfort for the patients during the urethral ultrasound study was when the Foley catheter was placed in the navicular fossa. A total of 96.6% of the patients would recommend urethral ultrasonography rather than cystoscopy or cystourethrography. A contrast-enhanced radiologic study of a normal urethra can be clearly seen in figure 2A; figures 2B, 2C, and 2D show ultrasound images of the anterior penile urethra, the posterior penile urethra, and the bulbous urethra. Discussion The age at which patients present with urethral stricture can vary. It has been observed that as the individual ages, the incidence of stricture is more frequent, with the greatest incidence in patients above the age of 55 years.1 The mean age of the patients in the present study was 66.4 years. According to statistics in the United States, stricture incidence for this age is calculated at 600/100,000. In relation to their etiology, strictures can be divided as follows: idiopathic, traumatic, infectious, and iatrogenic. Eighty percent of the patients studied had a previous TURP. In the study by Greenwell et al., the TURP was the cause of the stricture in 33% of the patients. According to a survey applied to urologists in the U.S., in relation to all procedures included in the questionnaire, dilation and urethrotomy are used to treat stricture in 92.8% and 85.6% of the cases, respectively. Of the 30 patients included in our study, 46.7% were managed with dilation and 23.3% had undergone a previous OIU. Cystourethrography is currently regarded as the study of choice for urethral stricture diagnosis. It offers the diagnostic advantages of complete visualization of the urethral course from the navicular fossa to the bladder neck in a retrograde phase and a micturition phase. Nevertheless, some disadvantages of cystourethrography are patient radiation exposure, the use of iodized contrast mediums, the changing of positions for the taking of images, the length of time of the study, the occasionally traumatic introduction of the catheter for instilling the contrast medium, the low sensitivity for observing strictures that are not significant, and the image interpretation variability. Moreover, stricture depth cannot be measured with this method and it often underestimates stricture length.5-8 Spongiofibrosis refers to the presence of fibrous tissue beyond the urethral epithelium that affects the spongy body, and in severe cases, the corpora cavernosa. The best treatment can be chosen if the amount of spongiofibrosis surrounding the stricture is known. Cystourethrography does not have the ability to show this periurethral tissue. Despite these disadvantages of cystourethrography, the reason why ultrasound is not a routine study in patients with urethral stricture or with urethral pathology in general is most likely related to the cost, the time it takes, and the lack of trained radiologists. Ultrasound that is carried out with a linear transducer, as was done in our study, has advantages over radiographic studies. It is possible to obtain real time longitudinal and transverse images, as well as objective measurements of the length and diameter of the urethral lumen. It is a study that is comfortable and well tolerated by patients and it does not require the use of ionizing radiation or contrast medium. To the contrary, the urethra can be irrigated with 184 P. Cruz García-Villa et al Figure 2 A) Cystourethrography with no data of urethral stricture. B) C) and D) Portions of the urethra (penile and bulbous) with adequate compliance and no evidence of stricture. physiologic solution or gel can be used to obtain adequate visibility. Perhaps one of the limitations of ultrasonography is the impossibility to observe the posterior urethra. In 1988, McAninch et al. first demonstrated the poor correlation between cystourethrography and ultrasound imaging for measuring stricture length. They showed how cystourethrography underestimated the length of the narrowings when compared with the length measured during the surgical procedure, whereas the ultrasound measurements coincided with the latter.9 Other studies followed, reporting their preliminary experience when evaluating urethral stricture with ultrasonography.10,11 In 1990 Merkle and Wagner predicted the success of the OIU in relation to the evaluation of scar tissue observed with ultrasound and found that 80% of the patients with ultrasonographic evidence of periurethral fibrosis had recurrences at 6 months from the surgery.12 Urethral ultrasound has various applications and one of them is in presurgical planning, especially for strictures located in the bulbous urethra. The evaluation of the length of the narrowing is perhaps the most important criterion for determining the best treatment.13 According to a study by Nash et al. in 1995, the length of the narrowing in the bulbous urethra observed through ultrasound was very highly correlated with the length found during the surgical procedure (p<0.007), which was not the case with retrograde urethrography.7 Another application of ultrasound is in severe strictures that are generally produced by perineal or pelvic trauma in which the size of the fibrosis or stricture of the bulbous urethra cannot be exactly defined through radiologic techniques.14 In our study, bulbar strictures presented in 40% of the patients, whereas penile strictures presented in 36.7%. This coincides with another study in which bulbar strictures were more frequent, presenting in 48.47% and penile strictures in 25.4%.15-17 The healthy urethral wall has special characteristics such as elasticity, softness, and the capacity to dilate when a liquid is instilled inside it (compliance). In ultrasound imaging, spongiofibrosis is seen as a thickened and irregular tissue with little compliance, projecting into the urethral lumen. This fibrosis can be observed with increased echogenicity, even though the areas that do not distend can alter the echogenicity. Unlike cystoscopy or cystourethrography, ultrasonography has the capacity to adequately measure the quantity of spongiofibrosis, which can be done by measuring the length and depth or by objectively measuring the diameter of the urethral lumen. During maximum retrograde Urethral ultrasound Figure 3 Stricture in the bulbous urethra with measurements of length and depth. distension, if the diameter of the urethral lumen measures less than 3 mm, the spongiofibrosis is regarded as severe. The presence of an acoustic shadow means that the fibrosis 185 is so dense that the ultrasound wavelengths cannot pass through it. In our study we measured the length of the strictures and the depth of the spongiofibrosis at their maximum points, obtaining a mean length of 0.84 ± 0.50 cm (0.20 to 2.27 cm) and a mean depth of 0.37 ± 0.1 cm (0.11 to 0.89 cm). We did not carry out a routine measurement of the urethral lumen, but we believe that it can be another objective parameter for stricture diagnosis.13 The mean length of the strictures found in our study was shorter than those reported in other series15,16 (fig. 3). When patients present with strictures that were already operated on, cystoscopic evaluation may be impossible. In those patients in whom a reconstruction with a scrotal skin flap was done, ultrasound imaging can even identify the presence of hair inside the urethra. Other applications of urethral ultrasound are the visualization of urethral stones, diverticula, abscesses, false pathways, and fistulas. In our series, we found a urethral diverticulum that was also diagnosed through cystourethrography (fig. 4). One of the limitations of our study was that not all patients had undergone cystourethrography and therefore we were not able to compare means. We believe that due to the complexity of the urethral pathology and its management, it is essential to have as much information on the stricture site as possible. Cystourethrography is a good diagnostic and detection method, but it does not provide all the information necessary for adequate treatment planning and choice. We join the other authors that have proposed that ultrasound study of the urethra be a complementary study in the evaluation and follow-up of patients with urethral stricture.13,15,18- 20 Figure 4 A) Cystourethrography with the image of a urethral diverticulum in the penile urethra. B) Ultrasonographic longitudinal view showing the diverticulum in the penile urethra. C) Transverse view in which the lumen of the diverticulum and the urethra can be seen. 186 Conclusions Urethral strictures present in men of all ages, with a greater incidence after 55 years of age. Urethral pathology is a frequent cause of visits to the urologist and represents an important expense for the patient and the institution. Urethral stricture treatment is complex and the majority of urologists opt for dilation or OIU, treatments that have an important recurrence rate. Urethroplasty in its different modalities is the best treatment in well-selected patients. Cystourethrography is regarded as the diagnostic study of choice, however it is not a perfect method. Urethral ultrasound is a noninvasive, inexpensive, and available method that provides objective information on the characteristics of the urethral stricture. We propose urethral ultrasound imaging as a complementary study to cystourethrography in all patients with urethral stricture. Conflict of interest The authors declare that there is no conflict of interest. Financial disclosure No financial support was received in relation to this article. References 1. Santucci RA, Jocye GF, Wise M. Male Urethral Stricture Disease J Urol 2007;177(5):1667-1674. 2. Romero Perez P, Mira Llinares A. Complications of the lower urinary tract secondary to urethral stenosis. Actas Urol Esp 1996;20(9):786-793. 3. Choudhary S, Singh P, Sundar E, et al. A comparison of sonourethrography and retrograde urethrography in evaluation of anterior urethral stricture. Clin Radiol 2004;59(8):736-742. 4. Gupta N, Dubey D, Mandhani A, et al. Urethral stricture assessment: a prospective study evaluating urethral ultrasonography and conventional radiological studies. BJU Int 2006;98(1):149153. P. Cruz García-Villa et al 5. Gupta S, Majumdar B, Tiwari A, et al. Sonography in the evaluation of anterior urethral strictures: Correlation with radiographic urethrography. J Clin Ultrasound 1993;21(4):231-239. 6. Das S. Ultrasonographic evaluation of urethral stricture disease. Urology 1992;40(3):237-242. 7. Nash PA, McAninch JW, Bruce JE, et al. Sonourethrography in the evaluation of anterior urethral strictures. J Urol 1995;154(1):72-76. 8. Morey AF, McAninch JW. Role of preoperative sonourethrography in bulbar urethral reconstruction. J Urol 1997;158(4):13761379. 9. McAninch JW, Laing FC, Jeffrey RB Jr. Sonourethrography in the evaluation of urethral strictures: a preliminary report. J Urol 1988;139(2):294-297. 10. Merkle W, Wagner W. Sonography of the distal male urethra -a new diagnostic procedure for urethral strictures: results of a retrospective study. J Urol 1988;140(6):1409-1411. 11. Gluck CD, Bundy AL, Fine C, et al. Sonographic urethrogram: comparison to roentgenographic techniques in 22 patients. J Urol 1988;140(6):1404-1408. 12. Merkle W, Wagner W. Risk of recurrent stricture following internal urethrotomy: prospective ultrasound study of distal male urethra. Br J Urol 1990;65(6):618-620. 13. Morey AF, McAninch JW. Sonographic staging of anterior urethral strictures. J Urol 2000;163(4):1070-1075. 14. Morey AF, McAninch JW. Ultrasound evaluation of the male urethra for assessment of urethral stricture. J Clin Ultrasound 1996;24(8):473-479. 15. Gong EM, Martinez Rios Arellano C, Chow JS, et al. Sonourethrogram to Manage Adolescent Anterior Urethral Stricture. J Urol 2010;184(4 Suppl):1699-1702. 16. Nuss GR, Granieri MA, Zhao LC, et al. Presenting Symptoms of Anterior Urethral Stricture Disease: A Disease Specific, Patient Reported Questionnaire to Measure Outcomes. J Urol 2012;187(2):559-562. 17. Greenwell TJ, Castle DE, Andrich JT, et al. Repeat Urethrotomy and Dilation for the treatment of Urethral Stricture are neither clinically effective nor Cost-Effective. J Urol 2004;172(1):275277. 18. Bullock TL, Brandes SB. Adult Anterior Urethral Strictures: A National Practice Patterns Survey of Board Certified Urologists in the United States. J Urol 2007;177(2):685-690. 19. Andrich DE, Mundy AR. Urethral Strictures and their surgical treatment. BJU Int 2000;86(5):571-580. 20. Selbold J, Werther M, Alloussi S, et al. Urethral Ultrasound as a Screening Tool for Stricture Recurrence after oral mucosa Graft Urethroplasty. Urology 2011;78(3):696-700. Rev Mex Urol 2013;73(4):187-190 ÓRGANO OFICIAL DE DIFUSIÓN DE LA SOCIEDAD MEXICANA DE UROLOGÍA www.elsevier.es/uromx Review Article Tumors of Cowper’s glands: a review of the literature A. Lisker-Cervantes, G. Romero-Vélez, C. I. Villeda-Sandoval, M. Sotomayor-de Zavaleta and R. Castillejos-Molina* Department of Urology, Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”, Mexico City, Mexico KEYWORDS Tumor; Cowper’s glands; Diagnosis; Treatment; Mexico. Palabras clave Tumor; Glándulas de Cowper; Diagnóstico; Tratamiento; México. Abstract The bulbourethral glands, or Cowper’s glands, originate as evaginations of the epithelium that cover the urogenital sinus. Their secretion neutralizes urine and lubricates the urethra prior to ejaculation. Occasionally, they can become infected or be the site of tumors or congenital disorders. Among the most frequently seen pathologies are congenital tumors, malignant tumors, and inflammatory processes. The first 2 should be considered when carrying out the physical examination. Correct diagnosis requires a high degree of suspicion and knowledge of this entity. Treatment should be individualized. Even though the authors state that resection is ideal for disease-free survival, conclusions about treatment cannot be made, given that there is insufficient information available on these disorders. Tumores de las glándulas de Cowper: una revisión de la literatura Resumen Las glándulas bulbouretrales o glándulas de Cowper se originan como evaginaciones del epitelio, que recubre el seno urogenital. Su secreción neutraliza la orina, además de lubricar la uretra previo a la eyaculación. Ocasionalmente, pueden infectarse o ser asiento de neoplasias o trastornos congénitos. Entre los trastornos vistos con mayor frecuencias están los tumores congénitos, tumores malignos y procesos inflamatorios. Los 2 primeros deben considerarse cuando se realiza la exploración física. El diagnóstico correcto requiere de un alto grado de sospecha y el conocimiento de esta entidad. El tratamiento debe ser individualizado. Aunque los autores concluyen que la resección es óptima para la sobrevida libre de enfermedad, no se pueden realizar conclusiones sobre el tratamiento basados en la información disponible. * Corresponding author at: Vasco de Quiroga N° 15, Colonia Sección XVI, Delegación Tlalpan, C.P. 14000, México D.F., México. Telephone: 5487 0900, ext. 2163. Fax: 5485 4380. Email: rcastillejos@hotmail.com (R. Castillejos-Molina). 0185-4542 - see front matter © 2013. Revista Mexicana de Urología. Publicado por Elsevier México. Todos los derechos reservados. 188 Introduction The bulbourethral glands originate as evaginations of the epithelium that cover the urogenital sinus. Their secretion neutralizes urine in addition to lubricating the urethra prior to ejaculation.1,2 They are called Cowper’s glands after William Cowper, who first described them in 1699. Diseases affecting these glands are rarely identified; however, they can be host to infections, tumors, and congenital disorders.2,3 The most frequently isolated microorganisms are Escherichia coli, Neisseria gonorrhea and Chlamydia trachomatis.1 Congenital tumors Syringocele consists of a cystic dilation of the Cowper’s glands and is a rare congenital anomaly. Nevertheless, according to Watson et al.,4 it is becoming more common. And according to Maziels et al., these lesions were formerly classified into 4 different groups: simple, perforated, imperforate, and ruptured.5 The recent medical literature suggests a classification based on 2 groups: open and closed, depending on their communication with the urethral lumen.4 These lesions are most commonly found in the pediatric population, with only 11 cases reported on in adults.6 According to Bevers et al., syringoceles in adults are lesions that are acquired secondarily to infection and trauma.7 Open syringoceles present with dysuria, urinary frequency, incontinence, terminal dripping, and hematuria, whereas closed syringoceles present with infravesical obstruction. Differential diagnosis should be made with synechiae, diverticula, valves, and periurethral abscesses, due to the not very specific syringocele symptomatology.6,8 Melquist et al. suggest a diagnostic algorithm based on a review of the literature.8 The first imaging study recommended for syringocele evaluation is transrectal ultrasound (TRUS), followed by cystourethrography (CUG). Closed syringoceles look like cystic lesions in the TRUS and the open ones appear to be filling defects when observed in the CUG.6-8 Magnetic resonance imaging (MRI) and computed axial tomography (CAT) can also be useful in evaluating closed syringoceles.4,8 Follow-up is adequate treatment, given that the symptomatology can improve without interventions.7,8 If the symptomatology persists, the patient can undergo endoscopic and open treatments. Endoscopic deroofing of the cysts has been reported with good results. 4,7,8 When endoscopic treatment fails, conduit ligature or open glandular excision are options.8,9 Malignant tumors Primary carcinomas of the bulbourethral glands are extremely rare, with only 21 reported cases in the medical literature. Adenocarcinomas are the predominant histopathologic type with 17 reported cases3,10-13; the rest are cases of cystadenocarcinoma.2,14-16 The last case was reported in 2003 by Hitsamatu et al.14 Due to their low incidence rate there is not enough information available to characterize these tumors. A. Lisker-Cervantes et al The clinical presentation varies in each of the reported cases (table 1). Syringocele is more common in the sixth decade of life and the patients did not report any other comorbidity. The majority presented with lower urinary tract symptoms that progressed to acute urine retention, or as a painful perineal tumor. The incidental finding of this tumor has also been reported; 2 during rectal examination and another during flexible urethroscopy.2,12,15 It has been described that in the rectal examination a petrous tumor can be delimited separate from the prostate. Bourque et al. suggest that syringocele should be suspected in patients presenting with painful tumors in the perineum or with incidental findings of narrowings in the bulbous or membranous urethra.10 Prostate-specific antigen (PSA) was introduced in 198017 and so its value was not reported in all the cases; however, when it was, the value was not elevated. Those cases before 1980 reported normal values of prostatic acid phosphatase (PAP). PSA and other laboratory studies are not diagnostic, but they can be useful in the differential diagnosis of prostate tumors. There is no laboratory finding that helps evaluate these tumors. CUG was used during the initial evaluation in the first cases but it was not useful in the majority of them.11 Bourque reported a narrowing of the posterior urethra that led to the suspicion of Cowper’s gland involvement.10 One of the cases was an incidental finding during a urethroscopy study. Cystoscopy in another patient produced no important findings, only extrinsic compression of the bulbous urethra.11 Small et al. used TRUS in their report and demonstrated a large, hypoechogenic cystic tumor inferior to the apex of the prostate that was later staged through CAT and MRI.15 We believe that TRUS is an adequate study for the initial approach, nevertheless, CAT or MRI should be considered for completing the evaluation, because they can provide more information with respect to extension and surgical planning. The final diagnosis will depend on the pathology study. Cowper’s glands are tubuloalveolar glands that are covered by a pseudo-stratified epithelium. Immunohistochemistry is positive for high molecular weight cytokeratin, mucin, and actin, whereas it is negative for PSA and PAP.1,14 Complete tumor excision is performed in the majority of patients. Excision extension varies depending on each case, from tumorectomy to pelvic exenteration. The adjuvant use of 5-fluorouracil (5-FU) was reported by Keen et al.3 with no benefits, whereas Hisamatu et al.14 used cisplatin and epirubicin together with radiotherapy and reported symptomatology improvement and non-quantified tumor reduction. Radiotherapy results vary depending on the regimen reported, as well as on clinical stage.2,3,14,15 Bourque et al.10 determined that these tumors are not hormone-dependent, making orchiectomy useless. The majority of authors agree that surgical treatment offers the best results. The reports do not conclude whether these tumors are aggressive or not. The majority of information has been extrapolated from cystic adenocarcinomas in the head and neck.14 As previously mentioned, surgical excision is the best treatment when the tumors are localized; a survival period of 13 years was reported for one of the patients.2 Not all the reports describe metastatic extension, but metastasis was reported in at least 6 out of 21 patients.10,11,14 Those patients have a worse outcome, with a 2-year survival period. Tumors of Cowper’s glands: a review of the literature 189 Table 1 Characteristics of the patients with adenocarcinoma and cystic adenocarcinoma of the Cowper’s glands Author Year Clinical presentation Tumor Treatment Commentary Paquet et al. 1884* Sur un cas d’epithélioma de la glande Cowper. J de l’anat. Et de la physiol. Pitrzikowski E, et al. 1885* Ein Fall von primären Carcinom der Cowperschen Drüsen. Ztschr. F. Heilk Blanc W, et al. 1910* Cancer of Cowper’s glands. La Loire Mèdicale Di Maio G 1928* Primary carcinoma of Cowper’s gland. Gazz. D’osp. Uhle CA, et al. 1935* Primary carcinoma of Cowper’s gland. J. Urol Gutierrez R 1937* Primary carcinoma of Cowper’s gland. Surg., Gynec. & Obst. Griseau WA, et al. 1951* Carcinoma of Cowper’s gland. J. Urol Urteaga OB, et al. 1956* Adenocarcinoma of Cowper’s glands. Arch. Peru. Pat. Clinic Marshall VF, et al. 1957* Carcinoma of Cowper’s gland. J. Urol Le Duc E 1962* Carcinoma of Cowper’s gland, report of the eleventh case. Calif. Med. Tomoyoshi T, et al. 1967* Adenocarcinoma of the Cowper’s gland. Acta. Urol. Jap. Derrick FC, et al. 1968* Cowper’s gland carcinoma. Report of a case. J.S. Carolina Med. Ass. Arduino LJ, et al. 1969 Carcinoma Prostatism En bloc excision DF at 30 months Bourque JL, et al. 1970 Adenocarcinoma Perineal pain, AUR En bloc excision Radiotherapy Symptomatic metastases 2-year survival Keen MR, et al. 1970 Adenocarcinoma Hematuria, AUR, perineal tumor Radiotherapy Chemotherapy (5FU) First chemotherapy 1.5-year survival Carpenter AA, et al. 1971 Cystic adenocarcinoma Prostate tumor Tumor excision Radiotherapy DF at 13 years Small JD, et al. 1992 Cystic adenocarcinoma Prostate tumor, LUTS Pelvic exenteration Radiotherapy Surgical support + radiotherapy Symptomatic metastases Follow-up loss (2 years) Steimberg S, et al. 1993 Adenocarcinoma LUTS, hematuria Urethrectomy + Chemotherapy (5FU) Madersbacher S, et al. 2001 Adenocarcinoma Recurrent pyelonephritis RRP Posterior ureterectomy Bladder exstrophy DF at 5 years Trnski D, et al. 2003 Cystic adenocarcinoma AUR TURB and tumorectomy DF at 6 months Hisamatsu H, et al. 2003 Cystic adenocarcinoma Perineal pain, rectal tumor Radiotherapy Chemotherapy (cisplatin-epirubicin) Pulmonary metastases 5-year survival DF: disease-free; AUR acute urine retention; 5FU: 5-Fluorouracil; LUTS: lower urinary tract symptoms; RRP: radical retropubic prostatectomy; TURB: transurethral resection of the bladder. *Cases compiled by Bourque JL, et al.10 Conclusions Tumors of the Cowper’s glands are rare. The correct diagnosis requires a high degree of suspicion and knowledge of this entity. There are no defined algorithms and so treatment must be individual. Even though the authors state that resection is ideal for disease-free survival, no conclusions can be made in relation to treatment based on the existing data. It is necessary to publish more information and the urologist must be aware of the diseases involving these glands. Conflict of interest The authors declare that there is no conflict of interest. 190 Financial disclosure No financial support was received in relation to this article. References 1. Chughtai B, Sawas A, O´Malley RL, et al. A neglected gland: a review of Cowper`s gland. Int J Andrology 2005;28:74-77. 2. Carpenter AA, Bernardo JR. Adenoid cystic carcinoma of Cowper`s gland: case report. J Urology 1970;106:701-703. 3. Keen MR, Golden RL, Richardson JF, et al. Carcinoma of Cowper`s gland treated with chemotherapy. J Urol 1970;104:854-859. 4. Watson RA, Lassoff MA, Sawczuk I, et al. Syringocele of Cowper`s gland duct: an increasingly common rarity. J Urology 2007;178:285. 5. Maizels M, Stephens FD, King LR, et al. Cowper’s syringocele: a classification of dilatation of Cowper’s gland duct based upon clinical characteristic of 8 boys. J Urol 1983;129:111-114 6. Kumar J, Kumar A, Babu N, et al. Cowper’s syringocele in an adult. Abdom Imaging 2007;32:428-430. 7. Bevers RFM, Abbekerk EM, Boon TA. Cowpers syringocele: Symptoms, classification and treatment of an unappreciated problem. J Urol 2000;163:782-784. A. Lisker-Cervantes et al 8. Melquist J, Sharma V, Sciullo D, et al. Current Diagnosis and Management of Syringocele: A Review. Intl Braz J Urol 2010;36(1):3-9. 9. Santin BJ, Pewitt EB. Cowper’s duct ligation for treatment of dysuria associated with Cowper’s syringocele treated previously with transurethral unroofing. Urology 2009;73(3):681. 10. Bourque JL, Charghi A. Primary carcinoma of Cowper`s gland. J Urol 1970;103:758-761. 11. Arduino LJ, Nuesse WE. Carcinoma of Cowper`s gland: Case report. J Urol 1969;102:224-229. 12. Madersbacher S, Treuthardt C. Paraurethral gland carcinoma in a man with bladder exstrophy diagnosed 41 years after bladder plate resection. J Urol 2001;166:2306-2307. 13. Steimberg S, Daneil A, Varcasia DA, et al. Adenocarcinoma de la glándula de Cowper. Revista Argentina de Urología 1993;58(4):177-179. 14. Hisamatsu H, Sakai H, Igawa T, et al. Adenoid cystic carcinoma of Cowper`s gland. BJU International 2003;91:1-2. 15. Small JD, Albertsen PC, Graydon JR, et al. Adenoid cystic carcinoma of Cowper`s gland. J Urol 1992;147:699-701. 16. Trnski D, Custovic Z, Soric T, et al. Primary adenoid cystic carcinoma arising in the region of Cowper`s gland. BJU International 2003;91:1. 17. De Angelis G, Rittenhouse HG, Mikolajczyk SD, et al. Twenty years of PSA: from prostate antigen to tumor marker. Rev Urol 2007;9(3):113-123. Rev Mex Urol 2013;73(4):191-194 ÓRGANO OFICIAL DE DIFUSIÓN DE LA SOCIEDAD MEXICANA DE UROLOGÍA www.elsevier.es/uromx Review article A new theory on albumin glomerular filtration and its tubular reabsorption: disputing the charge selectivity theory B. Condado-Arenas* and G. Pascual-Macfú School of Medicine and Health Sciences, Instituto Tecnológico y de Estudios Superiores de Monterrey, Monterrey, N. L., Mexico KEYWORDS Radical Glomerular sieving coefficient; Charge selectivity; Tubular reabsorption of albumin; Albuminuria mechanisms; Glomerular filtration; Mexico. Palabras clave Coeficiente de tamizaje glomerular; Selectividad por cargas; Reabsorción tubular de albúmina; Mecanismos de albuminuria; Filtración glomerular; México. Abstract The aim of this article is to demonstrate that the glomerular charge selectivity theory, which has been the basic tenet in the field of nephrology, is an erroneous concept, given that there are other mechanisms different from those proposed in that theory, such as albumin reabsorption at the proximal tubule. That the glomerular capillary wall is permeable to albumin through the novel glomerular sieving coefficient for albumin, an aspect that has been described in different studies, is also shown. This evidence has been compiled in the present article to demonstrate the veracity of the new theory of tubular reabsorption of albumin. Nueva teoría sobre la filtración glomerular de albúmina y su reabsorción tubular: refutado de la teoría de la “selectividad por cargas” Resumen El presente artículo tiene la finalidad de demostrar que la teoría de “selectividad por cargas” del glomérulo -la cual ha constituido un principio básico en el campo de la nefrología-, es un concepto erróneo, ya que diversos mecanismos diferentes a los planteados en esta teoría se llevan a cabo, tales como la reabsorción de albúmina en el túbulo proximal. También se demuestra que, la pared capilar glomerular es permeable a la albúmina. Esto se expuso mediante el nuevo coeficiente de tamizaje glomerular para albúmina, que se prueba mediante diversos estudios. En el presente escrito se recopilan estas evidencias para probar la veracidad de la nueva teoría de reabsorción tubular de albúmina. * Corresponding author at: Privada Carmelita N° 1600, Interior 464, Colonia Loma Larga, Monterrey, N. L., México. Telephone: (81) 1537 4423. Email: bernardocondadoarenas@gmail.com (B. Condado-Arenas). 0185-4542 - see front matter © 2013. Revista Mexicana de Urología. Publicado por Elsevier México. Todos los derechos reservados. 192 Introduction When glomerular filtration capacity is studied, it is commonly understood that the capillary wall has this capacity due to its ability to filter molecules according to their size and charge.1-6 Many studies have come to conclusions in favor of the theory of “charge selectivity”, based on research using anionic polysaccharides; however, recent studies have shown that the electrical repulsion of some anionic polysaccharides by the glomerular capillary wall is not equivalent, and therefore does not explain the apparent low selectivity for albumin.7 The present article attempts to explain the glomerular filtration of albumin as a more complex system in which there is no filtration by charges, showing that albumin passes through the glomerular capillary wall after being taken up and processed by the proximal tubular cells. 1,3,7-10 This theory has not yet been accepted;9 it is a recent one that provides a more effective explanation as to why changes in capillary wall permeability result in massive changes in albumin excretion in nephrotic ranges. The role of the glomerular capillary wall in the development of albuminuria The processes leading to albuminuria are complex and they involve hemodynamic, tubular absorption, and diffusion gradient elements. A description of the factors directly related to the glomerular capillary wall is presented herein. The glomerular capillary wall is made up of endothelial cells, the basal membrane, and the visceral epithelium.1,2,10 Historically, the basal membrane has been recognized as the structure that plays the most important role in the process of glomerular filtration. It is now sustained that podocytes have a more relevant role in this process. The main components of the glomerular basal membrane are type IV collagen, proteoglycans, and laminins. Proteoglycans are heterogeneous molecules composed of a protein that functions as a nucleus to which negatively charged lateral glycosaminoglycan chains are bound.10 This is the basis of the theory of filtration through charges. Nevertheless, in studies on transgenic mice that do not have the lateral chains of the main proteoglycan, heparan sulfate, these mice do not develop proteinuria,11-13 showing that the charges are not important in the filtration.11 One of these studies using agrin mutant mice showed that the loss of agrin, the molecule that binds to laminin, dystroglycan, and integrin receptors in the podocytes, also resulted in the loss of heparan sulfate at the level of the glomerular basal membrane and, in turn, the basal membrane charge, but it showed no effect at all on glomerular filtration.12 Another study on mutant mice in which the loss of negative charges in the basal membrane was achieved showed no difference in the excretion of the negatively charged substance, Ficoll®, with respect to the control mice.13 Another study in which mice were mutated to lose heparan sulfate showed that with the loss of this proteoglycan, contrary to expectations, no protein was found in the urine, and there were no manifestations of renal dysfunction even after 18 months.11 Podocytes play an important role in the development of albuminuria and nephrotic syndrome.14 Effacement of the B. Condado-Arenas y G. Pascual-Macfú podocyte foot processes is a common characteristic in proteinuric diseases.2,10 Different studies have been conducted that show severe proteinuria in genetic deficiencies of certain podocyte components.10 A study showed that rats injected with puromycin aminonucleoside developed massive proteinuria. Through electron microscopy it was discovered that the glomerulus displayed loss of the podocyte foot processes and that they had been replaced by epithelial cytoplasm.15 Current findings for the glomerular sieving coefficient for albumin The glomerular sieving coefficient (GSC) for albumin refers to the ratio of albumin concentration in Bowman’s space to the albumin concentration in plasma.8 The GSC is directly proportional to the concentration of a certain molecule in Bowman’s space and inversely proportional to the concentration of that same molecule in the plasma. For a freely filtered solute, the GSC is equal to one, whereas the GSC is equal to zero for a completely rejected solute. The total GSC corresponds to the sum of the individual GSCs for each layer; in other words, the total GSC corresponds to the sum of a GSC for the endothelium, a GSC for the basal membrane, and a GSC for the epithelium.16 This implies that an increase in the GSC of albumin, and thus of albuminuria, could be the result of a change in the GSC of the basal membrane, in the GSC of the endothelium, or in the GSC of the epithelium. Different studies with renal micropuncture have suggested that the GSC for albumin is 0.0006.17 The selectivity by size of the inert molecules that are similar in size to albumin gives them a GSC of 0.01-0.118. It is believed that the difference between the GSC of these molecules and that of albumin is explained by the “charge selectivity”. Different studies have analyzed the interaction of albumin with the glycoaminoglycans, using the 2-photon microscopy method. These studies concluded that the repulsive electrostatic interactions and the bases of “charge selectivity” do not exist under physiologic conditions.18 This has been confirmed by similar studies with negatively charged polysaccharides, that again did not show “charge selectivity”.18 Another study with negatively charged dextran sulfate showed that it is desulfated by a cellular mechanism during its filtration and that this desulfation was responsible for the differences in dextran sulfate sieving compared with non-charged dextran,19 which had given erroneous results in previous studies. However, studies continue to be carried out whose results favor the theory of “charge selectivity” and they assert that adequate methods were used in other previous studies. 20,21 An important study conducted by Russo and Comper (the 2 main proponents of the theories presented herein), et al. in 2007, established the new parameters for describing the glomerular filtration process. The most significant finding of that study was that the GSC for marked albumin measured in non-proteinuric rats was 0.034, a value much higher than those previously reported. The study by Russo and Comper et al. posited other important explanations that will be discussed further ahead in this article. A new theory on albumin glomerular filtration and its tubular reabsorption: disputing the charge selectivity theory The finding in the study by Russo and Comper et al. that the GSC for albumin was 0.034 is of vital importance because it means that even though the glomerular capillary wall is a great albumin barrier, that barrier is not albumin-impermeable.8 This corresponds to previous observations that when the glomerular blood flow is detained, albumin can be seen in the tubular lumen,22 given that the function of the glomerular barrier depends on the maintenance of a normal blood flow.22 This would not occur if the GSC for albumin were 0.0006.8 A recently published article objectively criticized the first studies carried out through the renal micropuncture method and more recently through that of 2-photon microscopy.23 The first method turned out to be unreliable because it was easy to underestimate albumin concentration. The 2-photon microscopy method was more reliable. This study concluded that the effect of the GSC of albumin was superior to that established by the renal micropuncture method and was close to the result obtained by Russo and Comper et al. with the 2-photon microscopy method.23 Albumin reabsorption at the tubular level The urinary exit flow for a substance basically depends on 3 factors described by the following equation:9 Urinary exit flow for albumin = filtration + secretion – reabsorption. It is logical that if under normal conditions there is no albuminuria, the right side of the equation should add up to zero. Stated differently, if it was previously maintained that albumin is filtered through the glomerular capillary wall and it is not secreted, then the value for filtration plus secretion would be above zero. Therefore it is logical that reabsorption plays an important role in returning the equation to zero urinary albumin. The study conducted by Russo and Comper et al. establishes the fact that the filtered albumin must be reabsorbed again into the bloodstream and it appears that this occurs through a recovery that is carried out by the cells of the proximal tubule. This was primarily determined by the fact that in vivo and in the isolated kidney, albumin clearance remains fractional.7 Immunogold study showed that diabetes-induced rats had less albumin reabsorption by endosomes and lysosomes in the S1 segment of the proximal tubule, leading to albuminuria in the early stages of diabetes.24 The same study by Russo and Comper et al. presented evidence of the existence of an albumin recovery pathway in the proximal tubule cells. They observed cytoplasmic vesicles with large quantities of albumin that were fused to the basolateral plasma membrane, resulting in the release of albumin into the peritubular capillary. 7 Other studies have demonstrated that albumin is degraded during its renal passage, probably by cells of the tubule.25 This is correlated with the observation through 2-photon microscopy of the presence of charged albumin structures, and that they extend from the apical to the basolateral part of the proximal tubule cells. A study supporting these assertions has shown that the filtered albumin is returned to the bloodstream through a high capacity pathway that transports albumin. 26 This 193 pathway has been identified under physiologic conditions in vivo and in the perfused isolated kidney. This pathway is inhibited in the non-filtering kidney; that is to say, in the kidney whose glomerular flow is detained. This same study concluded that the majority of the albuminuric states are a consequence of the poor functioning of this pathway.26 Another study using radio-iodized albumin, that would be a disadvantage with respect to other studies, found that there were large quantities of albumin fragments in urine, 98% of which were highly degraded and 2% were intact.27 Nevertheless, a more recent alternative in accordance with a GSC of 0.034, suggests that the majority of the albumin is taken by this reuptake pathway that is mediated by HK-2 cells of the proximal tubule and returned to the bloodstream, approximately 95% intact.7,18,26 The other 5% is destined for lysosomal degradation and posterior urinary excretion, coinciding quantitatively with other studies.18,28 One of the characteristics of the nephrotic states is that the changes in albuminuria are very big, compared with those in other molecules that are the same size as albumin.8,17 The lack of change in the filtration of these molecules that are different from albumin suggests that the increase in albumin excretion is not a problem of permeability of the glomerular capillary wall, nor is it a problem of diffusion. This albumin excretion in the nephrotic state is associated with the partial inhibition of the reuptake and degradation pathways, and results in a net increase in the ratio of intact forms of excreted albumin.8,29 Conclusions Both theories can cause confusion due to the great difference between what Haraldsson refers to in his article on glomerular filtration and the new theory being posited now. The basic principle of “charge selectivity” was a very important concept in the field of nephrology but recent physicochemical and renal clearance studies have shown that such a thing does not exist. And so it remains to be demonstrated that a normal glomerulus filters nephrotic levels of albumin, and if they are not reabsorbed, it will result in nephrotic ranges of albumin excretion.8 Therefore it is of the utmost importance that the tubule not be subjected to harmful processes, because in order for albuminuria to be present, there must be damage to the functions of the tubule that would prevent it from carrying out the normal albumin reabsorption process. However, further research and studies are necessary so that the theory described in this article is universally accepted and replaces what has been a fundamental principle of nephrology, the theory of “charge selectivity”. Conflict of interest The authors declare that there was no conflict of interest. Financial disclosure No financial support was received in relation to this article. 194 References 1. Kanwar YS, Linker A, Gist Farquhar M. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol 1980;86:688-693. 2. Alpers CE, Kumar V, Abbas AK, et al. The Kidney. In: Robbins and Cotran pathologic basis of disease. 8th Ed. Philadelphia: Saunders Elsevier; 2010. p. 905-969. 3. Comper WD, Haraldsson B, Deen WM. Resolved: normal glomeruli filter nephrotic levels of albumin. J Am Soc Nephrol 2008;19:427-432. 4. Bohrer MP, Baylis C, Humes HD, et al. Permselectivity of the glomerular capillary wall facilitated filtration of circulating polycations. J Clin Invest 1978;61:72-78. 5. Chang RLS, Deen WM, Robertson CR, et al. Permselectivity of the glomerular capillary wall: III restricted transport of polyanions. Kid Int 1975;8:212-218. 6. Rennke HG, Patel Y, Venkatachalam MA. Glomerular filtration of proteins: clearance of anionic, neutral, and cationic horseradish peroxidase in the rat. Kid Int 1978;13:278-288. 7. Russo LM, Sandoval RM, McKee M, et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kid Int 2007;71:504-513. 8. Comper WD, Russo LM. The glomerular filter: an imperfect barrier is required for perfect renal function. Curr Opin Nephrol Hypertens 2009;18:336-342. 9. Gekle M. Renal albumin handling: a look at the dark side of the filter. Kid Int 2007;71:479-481. 10. Patrakka J, Tryggvason K. New insights into the role of podocytes in proteinuria. Nat Rev Nephrol 2009;5:463-468. 11. Rossi M, Morita H, Sormunen R, et al. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J 2003;22:236-245. 12. Harvey SJ, Jarad G, Cunningham J, et al. Disruption of glomerular basement membrane charge through podocyte-especific mutation of agrin does not alter glomerular permselectivity. Am J Pathol 2007;171:139-152. 13. Goldberg S, Harvey SJ, Cunningham J, et al. Glomerular filtration is normal in the absence of both agrin and perlecan-heparan sulfate from the glomerular basement membrane. Nephrol Dial Transplant 2009;24:2044-2051. 14. Tryggvason K, Patrakka J, Wartiovaara. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 2006;354:1387-1401. B. Condado-Arenas y G. Pascual-Macfú 15. Graeme BR, Morris JK. An ultrastructural study of the mechanisms of proteinuria in aminonucleosidenephrosis. Kid Int 1975;8:219-232. 16. Deen WM. What determines glomerular capillary permeability? J Clin Invest 2004;114:1412-1414. 17. Tojo A, Endou H. Intrarenal handling of proteins in rats using fractional micropuncture technique. AJP Renal Physiol 1992;263:601-606. 18. Comper WD, Hilliard LM, Nikolic-Paterson DJ, et al. Diseasedependent mechanisms of albuminuria. Am J Physiol Renal Physiol 2008;295:1589-1600. 19. Comper WD, Tay M, Wells X, et al. Desulphation of dextran sulphate during kidney ultrafiltration. Biochem J 1994;297:3134. 20. Ohlson M, Sörensson J, Haraldsson B. Glomerular size and charge selectivity in the rat as revealed by FITC-ficoll and albumin. Am J Physiol Renal Physiol 2000;279:84-91. 21. Deen WM, Lazzara MJ, Myers BD. Structural determinants of glomerular permeability. Am J Physiol Renal Physiol 2001;281:579-596. 22. Graeme BR, Morris JK. Distribution of endogenous albumin in the rat glomerulus: Role of hemodynamic factors in glomerular barrier function. Kid Int 1976;9:36-45. 23. Tanner GA. Glomerular sieving coefficient of serum albumin in the rat: a two-photon microscopy study. Am J Physiol Renal Physiol 2009;296:F1258-F1265. 24. Tojo A, Onozato M, Ha H, et al. Reduced albumin reabsorption in the proximal tubule of early-stage diabetic rats. Histochem Cell Biol 2001;116:269-276. 25. Osicka TM, Pratt LM, Comper WD. Glomerular capillary wall permeability to albumin and horseradish peroxidase. APSN 1996;2:199-212. 26. Eppel GA, Osicka TM, Pratt LM, et al. The return of glomerular filtered albumin to the rat renal vein. Kid Int 1999;55:18611870. 27. Gudehithlu KP, Pegoraro AA, Dunea G, et al. Degradation of albumin by the renal proximal tubule cells and the subsequent fate of its fragments. Kid Int 2004;65:2113-2122. 28. Park CH, Maack T. Albumin absorption and catabolism by isolated perfused proximal convoluted tubules of the rabbit. J Clin Invest 1984;73:767-777. 29. Greive KA, Nikolic-Paterson DJ, Guimaraes MAM, et al. Glomerular permselectivity factors are not responsible for the increase in fractional clearance of albumin in rat glomerulonephritis. Am J Pathol 2001;159:1159-1170. Rev Mex Urol 2013;73(4):195-199 ÓRGANO OFICIAL DE DIFUSIÓN DE LA SOCIEDAD MEXICANA DE UROLOGÍA www.elsevier.es/uromx Clinical case Urinary incontinence management with artificial urinary sphincter following radical prostatectomy G. Fernández-Noyolaa,*, S. Ahumada-Tamayoa, J. Á. Martíneza, A. J. Camacho-Castro, F. García-Salcidoa, E. Muñoz-Ibarraa, G. Garza-Sainza, E. Mayorga-Gómeza, V. OsornioSáncheza, V. Cornejo-Dávilaa, A. Palmeros-Rodrígueza, I. Uberetagoyena-Tello de Menesesa, M. Cantellano-Orozcoa, G. Morales-Montora, C. Martínez-Arroyoa, R. W. SantaCruzb and C. Pacheco-Gahblera a Department of Urology, Hospital General “Dr. Manuel Gea González”, Mexico City, Mexico b Kendall Regional Medical Center, Miami, FL, USA KEYWORDS Urinary incontinence; Artificial sphincter; Radical prostatectomy; Radiotherapy; Mexico. Abstract The aim of this article is to present the technical aspects of placing the AMS-800™ artificial urinary sphincter for managing total postoperative urinary incontinence. A 73-year-old man with a past medical history of prostate cancer (CaP) underwent radical retropubic prostatectomy in the year 2000. The histopathologic report was stage pT4 adenocarcinoma of the prostate with a Gleason score of 4+5=9. He was managed with maximum androgen blockade and adjuvant radiotherapy, receiving a total of 112 Gy. After the radiotherapy, he presented with total urinary incontinence that required the use of 6 to 8 diapers daily. The patient underwent the placement of an AMS-800™ artificial urinary sphincter with no complications, obtaining total urinary continence and an important improvement in his quality of life. The management of urinary incontinence following radical prostatectomy with the AMS-800™ artificial urinary sphincter has been shown to be effective and is regarded as the gold standard by many urologists. The majority of patients using this device achieve urinary continence and their quality of life is significantly improved. * Corresponding author at: Calzada de Tlalpan N° 4800, Colonia Sección XVI, Delegación Tlalpan, C.P. 14080, México D.F., México. Telephone: 3624 5676, 4000 3044. Email: gerardofernandeznoyola@gmail.com (G. Fernández-Noyola). 0185-4542 - see front matter © 2013. Revista Mexicana de Urología. Publicado por Elsevier México. Todos los derechos reservados. 196 Palabras clave Incontinencia urinaria; Esfínter artificial; Prostatectomía radical; Radioterapia, México. G. Fernández-Noyola et al Esfínter urinario artificial para el manejo de la incontinencia urinaria posterior a prostatectomía radical Resumen Se expondrán los aspectos técnicos de la colocación del esfínter artificial AMS-800TM, para el manejo de la incontinencia urinaria total postoperatoria. Se presenta hombre de 73 años de edad, con antecedente de cáncer de próstata (CaP), postoperado de prostatectomía radical retropúbica en el año 2000, con reporte histopatológico de adenocarcinoma de próstata Gleason 4+5=9 pT4, por lo que se manejó con supresión androgénica máxima y radioterapia adyuvante, recibiendo en total 112 Gy. Posterior a la radioterapia inició con incontinencia urinaria total, que ameritó el uso de 6 a 8 pañales diarios. Se le colocó un esfínter urinario artificial AMS-800TM sin complicaciones, tras lo cual el paciente refiere una continencia urinaria total y una mejora importante en la calidad de vida. El manejo de la incontinencia urinaria posterior a prostatectomía radical con el esfínter artificial AMS-800TM ha demostrado ser efectivo, siendo considerado como el “gold standard” por muchos urólogos. Usando este dispositivo, la mayoría de los pacientes se encuentran sin pérdida urinaria, mejorando significativamente su calidad de vida. Introduction There are postoperative complications from the management of prostate cancer (CaP) with radical prostatectomy that can significantly deteriorate patient quality of life. One of these is urinary incontinence and it is a common symptom in patients that have been recently operated on. However, the majority of patients recover urinary continence, so much so, that one year after surgery this symptom persists in only 7% of the patients. In patients that receive adjuvant radiotherapy, the risk for urinary incontinence increases from 6% to 10%, depending on the dose and the modality employed.1-3 According to symptom frequency and the quality of life deterioration it causes, postoperative urinary incontinence can be classified as mild, moderate, or severe. The latter 2 significantly benefit from surgical treatment. The majority of authors agree that a postoperative follow-up period of at least one year is required before the final grade of incontinence can be determined.4-6 The idea of an artificial urinary sphincter was developed in the mid-twentieth century. In 1947, Foley designed the first artificial sphincter; it was a cuff that was inflated and deflated around the penis that was later developed as a surgical technique to be implanted around the urethra. The new era of the artificial urinary sphincters arrived in 1972 with Scott, Bradley, and Timm, with the elaboration of the AS-721™. This device required a laborious surgical act and had a high failure rate. The AMS-800™ artificial urinary sphincter has been used to treat moderate to severe urinary incontinence for 30 years. It has had excellent results with rates of 88% to 95% success at 5 years or more. The complication rates vary depending on the case series, and the most frequent complication is malfunction of the sphincter (11% to 23%), followed by system extrusion (8% to 20%), urethral erosion (8% to 10%), and infection (4% to 6%).7-9 In relation to the results of the artificial sphincter in patients that have received radiation therapy, a high incidence of urethral atrophy, erosion, and infection that has required surgical re-intervention has been reported, in comparison with those patients that have not undergone radiotherapy (41% vs. 11%). However, long-term continence and patient satisfaction do not appear to be affected by this modality.10-12 The persistence of stress incontinence can occur in more than 15% of the patients after artificial sphincter placement. This has been corrected by situating a more proximal cuff, or even by placing a second cuff, if system malfunction has been ruled out.12 Case presentation A 73-year-old man had a past medical history of CaP and underwent radical retropubic prostatectomy in 2000. The histopathologic report was prostate adenocarcinoma with a Gleason score of 4+5=9 and stage pT4 for which he was given maximum androgen blockade and adjuvant radiotherapy, receiving a total of 112 Gy. The patient presented with total urinary incontinence after the radiotherapy, requiring the use of 6 to 8 diapers daily. During his progression, he received multiple treatments with anticholinergics and serotonin reuptake inhibitors with no improvement. In the evaluation protocol, cystourethrography revealed a bladder capacity of 450 mL, as well as permeability of the entire urethra, total bladder emptying, and absence of the shadow of the urinary sphincter (fig. 1). Cystoscopy corroborated the lack of a functional sphincteric mechanism. The placement of an artificial urinary sphincter was proposed and the procedure was carried out with no complications. The patient was released on the second postoperative day. Eight weeks after surgery the sphincteric mechanism was activated and the patient achieved total urinary continence and an important improvement in his quality of life. Surgical technique After the placement of a bladder catheter and by the perineal approach, the bulbous urethra was located and dissected Esfínter urinario artificial para el manejo de la incontinencia urinaria posterior a prostatectomía radical 197 Figure 1 Cystourethrogram showing the absence of the shadow of the external urethral sphincter. Figure 2 Longitudinal midline incision, dissecting up to the urethra. Figure 3 Complete dissection of the bulbous urethra. Figure 4 Placement of the sphincter at the chosen site of the urethra, after having measured its diameter. (fig. 2), sparing the bulbocavernosus muscle as much as possible. The urethra was dissected at the level where the occlusive cuff was to be placed, until the dissector could pass the measuring tape through with ample space (fig. 3). Once the measuring tape was in place, the urethral circumference was measured, followed by the length of the cuff (fig. 4). The pressure-regulating balloon was then put in the prevesical space so that it barely lay over the muscle and the fascia through the suprapubic incision. Once the balloon was in position, it was filled with 22-23 cc of injectable solution. The connecting tube of this element was subcutaneously moved along until it exited at the level of the suprapubic incision, using the tubing passer that is one of the system components, and the 3 elements were connected (fig. 5). The control pump was placed in the scrotal sac in a subdartos pouch (fig. 6). The wounds were closed and the functioning of the mechanism and the urethral lumen occlusion were corroborated using a flexible cystoscope (fig. 7). The system was maintained inactive for 8 weeks after which it was then activated. This has reduced the infection rate and system extrusion. 198 A G. Fernández-Noyola et al B Figure 5 A and B Placement of the sphincter system components, first through suprapubic incision and then with the subcutaneous passage of the connecting tubes. A B Figure 6 The sphincter control pump is placed in the scrotum through a subdartos pouch. Conclusions Urinary incontinence management after radical prostatectomy with the AMS-800™ artificial urinary sphincter has been shown to be effective and is regarded as the gold standard by many urologists. Its placement is a simple procedure with low morbidity in the hands of the experienced surgeon and it provides the patient with satisfactory functional and esthetic results that significantly improve quality of life. Figure 7 A and B Cystoscopy identifies the open urethra without the effect of the sphincter, and then with the functioning sphincter. Esfínter urinario artificial para el manejo de la incontinencia urinaria posterior a prostatectomía radical Conflict of interest The authors declare that there is no conflict of interest. Financial disclosure No financial support was received in relation to this article. References 1. Fowler FJ, Barry MJ, Lu-Yao G, et al. Patient-reported complications and follow-up treatment after radical prostatectomy. The national Medicare experience: 1988-1990. Urology 1993;42(6):622-629. 2. Herr H. Quality of life of incontinent men after radical prostatectomy. J Urol 1994;151(3):652-654. 3. McCammon KA, Klom P, Main B, et al. Comparative quality of life analysis after radical prostatectomy or external beam radiation for localized prostate cancer. Urology 1999;54(3):509516. 4. Jonler M, Messing EM, Rhodes PR, et al. Sequelae of radical prostatectomy. Br J Urol 1994;74(3):352-358. 199 5. Donnellan SM, Duncan HJ, MacGregor RJ, et al. Prospective assessment of incontinence after radical retropubic prostatectomy: Objective and subjective analysis. Urology 1997;49(2):225-230. 6. Scott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by implantable prosthetic sphincter. Urology 1973;1(3):252-259. 7. Tomaschi W, Suster G, Holtl W. Bladder neck strictures after radical retropubic prostatectomy: Still an unsolved problem. Br J Urol 1998;81(6):823-826. 8. Chao R, Mayo ME. Incontinence after radical prostatectomy: Detrusor or sphincteric causes. J Urol 1995;154(1):16-18. 9. Meulen PH, Zambon V, Kessels AG, et al. Quality of life, functional outcome and durability of the AMS 800 artificial urinary sphincter in patients with intrinsic sphincter deficiency. Urol Int 2003;71(1):55-60. 10. Wilson SK, Delk JR 2nd, Henry GD, et al. New surgical technique for sphincter urinary control system using upper transverse scrotal incision. J Urol 2003;169(1):261-264. 11. Litwiller SE, Kim KB, Fone PD, et al. Post-prostatectomy incontinence and the artificial urinary sphincter: a long-term study of patient satisfaction and criteria for success. J Urol 1996;156(6):1975-1980. 12. Gomha MA, Boone TB. Artificial urinary sphincter for post-prostatectomy incontinence in men who had prior radiotherapy: a risk and outcome analysis. J Urol 2002;167(2 Pt 1):591-596. Rev Mex Urol 2013;73(4):200-203 ÓRGANO OFICIAL DE DIFUSIÓN DE LA SOCIEDAD MEXICANA DE UROLOGÍA www.elsevier.es/uromx Clinical case Crossed renal ectopia with fusion and multiple renal calculi managed with nephrectomy through the anterior paramedian approach F. R. Zamora-Varelaa, V. M. González-Tejedalb and A. González-Ambrizc a Urology Speciality Residency, Hospital Regional “Dr. Valentín Gómez Farías”, ISSSTE, Guadalajara, Jal., Mexico b Transplantation Administration, Hospital Civil “Dr. Miguel Silva”, Morelia, Mich., Mexico c Department of Urology, Hospital Civil “Dr. Miguel Silva”, Morelia, Mich., Mexico KEYWORDS Crossed renal ectopia; Renal fusion; Renal lithiasis; Mexico. Abstract Congenital renal anomalies are not common. Crossed renal ectopia (CRE) is the second most frequent abnormality after horseshoe kidney. Its diagnosis is usually incidental in the third or fourth decade of life or when the patient presents with urinary tract infections, hematuria, lithiasis, or renal-ureteral colic. The aim of this article was to present the case of a patient with the classic symptoms of renalureteral colic who was diagnosed with CRE with an inferiorly fused, non-functioning kidney secondary to multiple renal calculi, and to describe the management with nephrectomy through the extraperitoneal anterior paramedian approach. A 39-year-old man presented with a classic case of renal-ureteral colic and during his evaluation multiple renal calculi outside of the normal renal topography were found. An abdominal computed tomography (CT) scan was done and CRE was diagnosed. The non-functioning inferiorly fused kidney was revealed in the contrast-enhanced and three-dimensionally reconstructed CT, and management with nephrectomy was decided upon. We believe that the extraperitoneal anterior paramedian approach provides good access for this type of congenital anomaly, given the anterior location of the renal unit. * Corresponding author at: Paseo de las brisas N° 4128-302, Colonia Lomas Altas, C.P. 45128. Zapopan, Jal., México. Telephone: (33) 3749 3944. Email:dr.zamora@hotmail.com (F. R. Zamora-Varela). 0185-4542 - see front matter © 2013. Revista Mexicana de Urología. Publicado por Elsevier México. Todos los derechos reservados. Crossed renal ectopia with fusion and multiple renal calculi managed with nephrectomy through the anterior paramedian approach201 Palabras clave Ectopia renal cruzada; Fusión renal; Litiasis renal; México. Ectopia renal cruzada con fusión y litiasis múltiple, nefrectomía con abordaje paramedio anterior Resumen Las anomalías renales congénitas son poco frecuentes. La ectopia renal cruzada (ERC) es la segunda anomalía más frecuente tras el riñón en herradura. Su diagnóstico suele ser incidental hacia la tercera o cuarta década de la vida, o al cursar con infecciones de vías urinarias, hematuria, litiasis o cólico reno-ureteral. El objetivo es presentar el caso de un paciente que cursó con la sintomatología clásica de un cólico reno-ureteral, diagnosticándolo con ERC con fusión inferior y exclusión del riñón fusionado secundaria a litiasis múltiple, así como exponer el manejo mediante nefrectomía por abordaje paramedio anterior extraperitoneal. Se presenta paciente masculino de 39 años de edad, con cuadro clínico clásico de cólico renoureteral, durante su estudio se encontró litiasis múltiple fuera de la topografía normal renal, por lo que se realizó una tomografía computada (TC) abdominal mediante la cual se diagnosticó la ERC, se reconstruyó tridimensionalmente con contraste y se encontró la exclusión del riñón fusionado inferior, por lo cual se manejó con nefrectomía. Consideramos que el abordaje paramedio anterior extraperitoneal es un buen acceso para este tipo de anomalía congénita, debido a la situación anterior de la unidad renal. Introduction Crossed renal ectopia (CRE) with fusion represents 85% to 90% of the published cases and the most frequent variety is the unilateral fused kidney with inferior ectopia. CRE is defined as the position in the retroperitoneum different from the normal one, in which the kidney crosses the midline and is situated contralaterally to the side where its ureter normally inserts into the bladder. Diagnosis is incidental in the majority of cases and symptomatology is vague, simulating renal-ureteral colic in some cases. It is associated with urinary tract obstruction due to bridles or abnormal vascular supply to the ectopic kidney. It is also associated with other abnormalities such as vesicoureteral reflux, ureterocele, and anorectal malformation. Diagnosis is made through excretory urogram and contrast-enhanced three-dimensional computerized tomography (CT) and treatment is directed at the complications, more than at the anatomical anomaly, itself. Case presentation A 39-year-old man had illness onset 4 years earlier, with the clinical presentation of right renal-ureteral colic that was never managed by a physician. The patient self-medicated with anti-inflammatory agents for pain control. However, 7 months ago he presented with similar but more intense symptoms, to which were added fever, hematuria, and irritative urinary symptoms. He sought private medical attention and the physician ordered a simple abdominal x-ray and referred the patient to our hospital for management. He was first evaluated at the emergency room and was found to be in good general health. A complete blood count, blood chemistry, serum electrolytes, and urinalysis were ordered. The blood tests reported leukocytosis of 11,800, hemoglobin 12.2 g/dl, platelets 352,000, glucose 98, creatinine 0.8, and urea 17. The urinalysis showed a pH of 5.5, leukocyturia, erythrocyturia, and bacteriuria. The abdominal x-ray (fig. 1) revealed radiopaque images suggestive of stones measuring approximately 2 cm x 1.5 cm in the right lower quadrant above the iliac crest. An abdominal CT scan showed the absence of the left kidney and an enlarged right kidney with calcifications under the lower pole (fig. 2A); other views displayed an image that could correspond to an ectopic left kidney with multiple stones in its interior that was fused with the right kidney (figs. 2B and 2C). There was inadequate contrast material uptake by the fused ectopic kidney shown in the urotomographic reconstruction (fig. 3A). The patient was evaluated together with the transplantation service. An angiotomography scan (fig. 3B) revealed a single artery and vein and inadequate vascular supply to the ectopic kidney. Nephrectomy through the extraperitoneal paramedian approach was performed on the ectopic kidney. It was carried out with no complications and the total surgery duration was one hour 10 minutes. Blood loss was 500 cc. Nine stones measuring 2.5 cm x 1.5 cm were extracted from the surgical specimen. The patient was released from the hospital after 72 hours. Discussion Lithiasis in kidneys that have some type of anatomical alteration is a particularly great challenge for the urologist, due to the fact that the abnormal anatomy prevents the use of the same disintegration or extraction access routes that are utilized in normal kidney units.1 Embryologically, the definitive kidney originates at the fifth week of intrauterine life and its development depends on the chemical interaction of the ureteric bud near its joining with the continuous mass of non-differentiated mesenchymal cells called the metanephric blastema. This union ascends from its pelvic position toward the ipsilateral renal fossa, turning inwards on its longitudinal axis until the definitive renal-ureteral unit is formed during the following 3 202 F. R. Zamora-Varela et al Figure 1 X-ray showing the multiple renal calculi. weeks. Pelvic kidney, ectopic kidney, or renal malformation are explained by developmental defects in the migration stage.2,3 Normal fetal and embryonic development of the kidney can be altered by various factors that, in turn, are associated with other urinary tract malformations. A total of 35% to 40% of the congenital abnormalities are located in the genitourinary tract and 10% of all living beings are born with some type of urinary tract anomaly.2 A B The ectopic kidney is defined as one that is congenitally in a position different from its usual location in the lumbar region due to a flaw in the process of its ascent, and that crosses the midline and becomes situated on the opposite side from where it normally connects to the bladder.2,4 CRE is the second most frequent anomaly with fusion after horseshoe kidney. The first case was published by Pamarolus in 1654 and in 1957 it was classified by McDonald and McClellan.3,5 Their classification is the one currently in use: crossed ectopia with fusion, which makes up 85% of the cases; inferior crossed ectopia without fusion; solitary crossed ectopia; and bilateral crossed ectopia. The fused varieties are divided into: unilateral fused kidney with inferior ectopia; sigmoid or S-shaped kidney; L-shaped kidney; lump kidney; disc kidney; and unilateral fused kidney with superior ectopia.5-8 Various theories have been proposed, but the precise mechanisms by which CRE occurs are not known.5 Among them are the mechanical theory, the ureteral theory, the theory of biochemical stimuli-induced migration, the teratogenic theory, and the theory of the abnormal rotation of the caudal end of the developing fetus.3,5 CRE with fusion is a rare malformation with an incidence of 1:7,500; 6 it is more frequent in men with a ratio of 1.4:1,7 and the left-to-right ratio is 3:1.3 Its clinical presentation is asymptomatic in the majority of cases and it generally develops in the third or fourth decade of life.9 Because irrigation is different from the norm, kinking or compression of the urinary tract can cause ureteropelvic junction stricture, usually of the ectopic kidney, resulting in hydronephrosis with or without lithiasis in 9% of the cases,1-3,6 hematuria, non-specific abdominal pain, recurrent urinary infections, and renal-ureteral pain, among others.3 Its diagnosis is based on intravenous urography; contrastenhanced 3-dimensional CT is usually the best imaging technique for detailing the situation of the ectopic kidney.2,3,5,7,8 Treatment should be opportune and directed at the complications rather than at the congenital anomaly itself, and includes antibiotic prophylaxis, extracorporeal lithotripsy, C Figure 2 Computerized tomography scan. A) Left renal agenesis; calcifications are starting to be seen outside the right kidney. B) Image corresponding to the left ectopic kidney with multiple stones. C) Left kidney fusion. Crossed renal ectopia with fusion and multiple renal calculi managed with nephrectomy through the anterior paramedian approach203 A B Figure 3 A) Urotomography scan showing the left renal ectopia and the multiple stones in the fused kidney with no contrast medium uptake. B) Angiotomography scan showing inadequate vascularization of the crossed ectopic kidney. ureterolithotripsy, percutaneous nephrolithotomy, pyelolithotomy, pyeloplasty, and in cases of non-functioning kidney, nephrectomy.1,7 Conclusions Our patient presented with the classic symptoms of renalureteral colic and during his evaluation multiple renal calculi outside the normal kidney topography were found. Abdominal CT scan was done and CRE was diagnosed. Contrast-enhanced 3-dimensional reconstruction revealed a fused non-functioning kidney with inferior ectopia for which nephrectomy was performed. In our opinion, the extraperitoneal anterior paramedian approach is a good access route for this type of congenital anomaly, given the anterior situation of the renal unit. Conflict of interest The authors declare that there is no conflict of interest. Financial disclosure No financial support was received in relation to this article. References 1. Gupta M, Lee MW. Treatment of stones with complex or Anomalous Renal Anatomy. Urol Clin North Am 2007;34(3):431-441. 2. Montell Hernández OA, Vidal Tallet A. Pielonefritis en ectopia renal cruzada y fusionada. Presentación de caso. Revista médica electrónica 2010;32(4). 3. Sousa Escandón MA, González Rodríguez A, García Figueiras R, et al. Ectopia renal cruzada: posibilidades radiológicas de la TAC helicoidal. Actas Urol Esp 2002;26(5):313-319. 4. Lizado BJR, Godoy MJG. Ectopia renal simple. Informe de un caso y revisión de la literatura. Rev Med Hondur 2011;79(1):1921. 5. Aguilera Tubet C, Del Valle Schaan JI, Martín García B, et al. Tumor renal en ectopia renal cruzada con fusión. Actas Urol Esp 2005;29(10):993-996. 6. Durán Álvarez S, Guerra Rodríguez M, Díaz Zayas N, et al. Ectopia renal cruzada con fusión, reflujo vesicoureteral y riñón ectópico afuncional: informe de un caso. Rev Cub Pediatr 2010;8(1). 7. Aguilar-Cota JJ, Alvarado-García R, Ramón-Garrido J. Ectopia renal cruzada no fusionada con malformación anorrectal y ureterocele en un niño. Acta Pediatr Mex 2009;30(5)5:254-257. 8. Aysel T, Tülay O, Turhan C. Multidetector CT urography of renal fusion anomalies. Diagn Interv Radiol 2009;15(2):127-134. 9. Ghosh BC, DeSantis M, Kleyner Y, et al. Crossed Fused Renal Ectopia with Calculi. J Am Coll Surg 2008;206(4):753. Rev Mex Urol 2013;73(4):204-207 ÓRGANO OFICIAL DE DIFUSIÓN DE LA SOCIEDAD MEXICANA DE UROLOGÍA www.elsevier.es/uromx Clinical case Application of dorsal buccal mucosa graft for penile urethral stricture treatment: technical aspects G. Fernández-Noyolaa,*, S. Ahumada-Tamayoa, J. Á. Martíneza, A. J. Camacho-Castroa, F. García-Salcidoa, E. Muñoz-Ibarraa, G. Garza-Sainza, E. Mayorga-Gómeza, V. OsornioSáncheza, V. Cornejo-Dávilaa, A. Palmeros-Rodrígueza, I. Uberetagoyena-Tello de Menesesa, M. Cantellano-Orozcoa, G. Morales-Montora, C. Martínez-Arroyoa, E. A. Ramírez-Pérezb, J. C. López-Silvestreb and C. Pacheco-Gahblera a Department of Urology, Hospital General “Dr. Manuel Gea González”, Mexico City, Mexico b Centro de Cirugía Reconstructiva Uretral, Mexico City, Mexico KEYWORDS Urethral stricture; Urethroplasty; Buccal mucosa; Dorsal graft; Mexico. Abstract The aim of this article was to present the technical aspects of dorsal urethroplasty with buccal mucosa graft for penile urethral stricture treatment. A 46-year-old man had a past medical history of sexually transmitted infections from 14 years prior that were undocumented, but nevertheless resolved, with parenteral antibiotics. The patient had lower urinary tract symptomatology that began 10 years ago, with documented stricture of the penile urethra, managed through internal urethrotomy. Progression was satisfactory, but 2 years ago he presented once again with lower urinary tract symptoms and penile urethral stricture, for which a second internal urethrotomy was performed. One year later the patient was seen at our institution for symptom persistence. Cystourethrography revealed a 3 cm long stricture of the penile urethra. Urethroplasty was carried out with a dorsal buccal mucosa free graft with no complications and the patient was released on the second postoperative day. The transurethral catheter was removed 4 weeks later, after which the patient presented with adequate micturition and symptom remission. Dorsal urethroplasty with buccal mucosa free graft is an effective technique in long and complex stricture of the urethra and it should be regarded as the technique of choice for this type of urethral stricture. * Corresponding author at: Calzada de Tlalpan N° 4800, Colonia Sección XVI, Delegación Tlalpan, C.P. 14080, México D.F., México. Telephones: 4000 3044, 3624 5676. Email: gerardofernandeznoyola@gmail.com (G. Fernández-Noyola). 0185-4542 - see front matter © 2013. Revista Mexicana de Urología. Publicado por Elsevier México. Todos los derechos reservados. Application of dorsal buccal mucosa graft for penile urethral stricture treatment: technical aspects Palabras clave Estenosis uretra; Uretroplastía; Mucosa oral; Injerto dorsal; México. 205 Aplicación de injerto dorsal de mucosa oral para el tratamiento de la estenosis de uretra peniana. Aspectos técnicos Resumen Se expondrán los aspectos técnicos de la uretroplastía dorsal con injerto de mucosa oral, para el tratamiento de la estenosis de uretra peniana. Se presenta hombre de 46 años de edad, con antecedente de infecciones de transmisión sexual hace 14 años, la cual no se documentó, sin embargo resolvió con antibióticos parenterales. Inició hace 10 años con sintomatología del tracto urinario bajo, documentándose estenosis de uretra peniana manejada en medio externo con uretrotomía interna, evolucionando satisfactoriamente; sin embargo, hace 2 años presenta nuevamente sintomatología del tracto urinario bajo, evidenciándose nuevamente estenosis de uretra peniana, por lo cual se le realiza una segunda uretrotomía interna. Un año después acude a nuestra Institución por persistencia de la sintomatología, se realizó cistouretrografía observando una estenosis de la uretra peniana de 3 cm de longitud. Se realizó plastía de uretra con injerto dorsal libre de mucosa oral sin complicaciones, egresándose al segundo día de postoperatorio. Se retiró la sonda transuretral a las 4 semanas, tras las cuales presentó una adecuada micción y remision de la sintomatología. La uretroplastía dorsal con injerto libre de mucosa oral es una técnica efectiva en estenosis de uretra larga y compleja, que por los resultados obtenidos se le debe considerar como la técnica de primera elección en este tipo de estenosis uretrales. Introduction Management of complex stricture of the urethra has significantly changed in the last few years. Today, graft techniques are regarded as the first treatment of choice, given that there is a high 70% recurrence rate with internal urethrotomy. The good results obtained with dorsal urethroplasty with buccal mucosa free graft for the treatment of long and complex strictures at any level of the urethra have led to its being considered the gold standard of graft techniques. 1,3 The use of buccal mucosa for the treatment of urethral stricture was first described by Kasaby in 1993. In 1995 Guido Barbagli reported the technique of dorsal urethroplasty with mucosa free graft and since then longterm results have shown success rates of 92% to 97%. Then in 1996 Morey and McAninch described the technique with ventral buccal mucosa free graft for 2 cm to 5 cm bulbar strictures.4,5 Buccal mucosa has important advantages over skin as a graft material, that include its being available in all patients, it is a humid epithelium, it can be easily obtained, it lends itself to surgical manipulation, it can be thinned without damaging its vascularity, it has immunologic benefits making it less prone to infection, and it is more resistant to stricture recurrence. In addition, it has a submucosa with a dense capillary network that facilitates the prompt absorption of nutrients from the tissue bed, enabling early neovascularization. The dorsal approach is considered to be more favorable than the ventral or lateral approaches because at that level the corpus spongiosum is not as thick and the attachment of the buccal mucosa to the corpora cavernosa reduces graft contraction, making neovascularization easier and avoiding the risk for sacculation, fistulas, and stricture recurrence.6-9 Moreover, because the ventral approach lacks a rigid and stable support, the graft runs the risk of becoming sacculated, giving rise to post-micturition dribble, infections, and ejaculatory disorders.10 In regard to the site of choice for extracting the buccal mucosa to be grafted, it canbe obtained either in the region of the cheek anterior to Stensen’s duct or in the region of the inferior lip, depending on the urethral length required for the reconstruction. However, the majority of authors opt for the cheek mucosa because it has a better consistency and thus is easier to manipulate than the inferior lip.11,12 Case presentation A 46-year-old man had an undocumented past medical history of sexually transmitted infections 14 years prior that were resolved with parenteral antibiotics. Ten years ago he began to present with lower urinary tract symptoms that resulted in penile urethra stricture. He was managed with internal urethrotomy and progressed satisfactorily. However, 2 years ago the patient again presented with lower urinary tract symptoms and a recurrent stricture of the penile urethra for which he underwent a second internal urethrotomy. One year later he sought medical attention at our institution for symptom persistence. The study protocol was initiated and cystourethrography identified a stricture of the penile urethra (fig. 1). Urethroplasty with a dorsal buccal mucosa free graft was performed with no complications and the patient was released on the second postoperative day. The transurethral catheter was removed 4 weeks later and the patient then presented with adequate micturition and symptom remission. Surgical technique We began the procedure with urethrocystoscopy with placement of the hydrophilic guidewire to the bladder. Then 15 cc of methylene blue were instilled through the urethra and a 14Fr Nelaton catheter was placed at the stricture site and the skin was marked. A 5 cm midline perineal incision was 206 G. Fernández-Noyola et al Figure 1 Retrograde cystourethrogram that clearly identifies the narrowing of the anterior or penile urethra. Figure 2 Longitudinal midline incision in the perineum. Figure 3 Complete dissection of the anterior and posterior urethra up to the narrowing site. Figure 4 The taking of the buccal mucosa graft, with care not to injure any glands. Figure 5 Cleaning the graft and making the cut along its length and diameter. Figure 6 Graft placement and suturing on the strictured urethra. made and dissection by planes was done avoiding excessive manipulation of the urethra (fig. 2). The body of the penis was then inverted to expose it at the perineal incision (fig. 3). Mobilization of the urethra at the level of the left lateral surface was begun so its dorsal portion could be exposed, taking care to maintain a 45° angle between the corpus cavernosum and the mobilized urethra. A dorsolateral incision of the urethra was made along the entire stricture and simultaneously a 3 cm long buccal mucosa graft was taken from the right cheek, making sure not to injure the parotid duct (fig. 4). After that, the buccal mucosa was placed at the dorsolateral penile level, attaching it to the corpus cavernosum with Vicryl™ 5-0, joining the lateral edge of the buccal mucosa with the lateral end of the urethra. A 14Fr silicon Foley transurethral catheter was placed and the other lateral end of the urethra was then completely closed with Vicryl™ 5-0 (figs. 5 and 6). Conclusions Dorsal urethroplasty with buccal mucosa free graft is an effective technique for treating complex stricture of the penile urethra associated with moderate spongiofibrosis. In experienced hands, it is a relatively simple procedure with little morbidity. Given the results obtained with this approach in large case series, it should be regarded as the first-choice technique for this type of urethral stricture. Application of dorsal buccal mucosa graft for penile urethral stricture treatment: technical aspects Conflict of interest The authors declare that there is no conflict of interest. Financial disclosure No financial support was received in relation to this article. References 1. Berger B, Sykes Z, Freedman M. Patch graft urethroplasty for urethral stricture disease. J Urol 1976;115(6):681-684. 2. El-Kasaby AW, Fath-Alla M, Noweir AM, et al. The use of buccal mucosa patch in the management of anterior urethral strictures. J Urol 1993;149(2):276-278. 3. Grady JD, McCammon K, Schlossberg SM. Buccal mucosa graft for penile urethral strictures. J Urol 1999;161:375A. 4. Gupta NP, Ansari MA, Dogra PN, et al. Dorsal buccal graft urethroplasty by a ventral sagittal urethrotomy and minimalaccess perineal approach for anterior urethral stricture. BJU Int 2004;93(9):1287-1290. 207 5. Wessells H, McAninch JW. Use of free grafts in urethral stricture reconstruction. J Urol 1996;155(6):1912-1915. 6. Wessells H. Ventral onlay graft techniques for urethroplasty. Urol Clin North Am 2002;29(2):381-387. 7. Barbagli G, Palminteri E, Guazzoni G, et al. Bulbar Urethroplasty using buccal mucosa grafts placed on the ventral, dorsal or lateral surface of the urethra: are results affected by the surgical technique? J Urol 2005;174(3):955-957. 8. Elliot SP, Metro MJ, McAninch JW. Long-term followup of the ventrally placed buccal mucosa onlay graft in bulbar urethral reconstruction. J Urol 2003;169(5):1754-1757. 9. Barbagli G, Guazzoni G, Palminteri E, et al. Anastomotic fibrous ring as cause of stricture recurrence after bulbar onlay graft urethroplasty. J Urol 2006;176(2):614-619. 10. Eppley BL, Keating M, Rink R. A buccal mucosal harvesting technique for urethral reconstruction. J Urol 1997;157(4):1268-1270. 11. Tolstunov L, Pogrel MA, McAninch JW. Intraoral morbidity following free buccal mucosal graft harvesting for urethroplasty. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;84(5):480-482. 12. Ramirez P, López S, Pérez E, et al. Uretroplastia de mínima invasion con mucosa oral para el manejo de estenosis complejas de uretra anterior en un solo tiempo. Rev Mex Urol 2012;72(2):63-71. Rev Mex Urol 2013;73(4):208-211 ÓRGANO OFICIAL DE DIFUSIÓN DE LA SOCIEDAD MEXICANA DE UROLOGÍA www.elsevier.es/uromx Clinical case Management of post-radical prostatectomy male urinary incontinence with a transobturator sling (AdVance®) S. Ahumada-Tamayo*, R. Santa-Cruz, J. Á. Martínez, G. Fernández Noyola, F. GarcíaSalcido, E. Muñoz-Ibarra, A. J. Camacho-Castro, E. Mayorga-Gómez, V. Osornio-Sánchez, G. Garza-Saenz, V. Cornejo-Dávila, A. Palmeros-Rodríguez, I. Uberetagoyena-Tello de Meneses, C. Martínez-Arroyo, M. Cantellano-Orozco, J. G. Morales-Montor and C. Pacheco-Gahbler Department of Urology, Hospital General “Dr. Manuel Gea González”, Mexico City, Mexico KEYWORDS Stress urinary incontinence; Post-radical prostatectomy; Transobturator sling; Mexico. Abstract Urinary incontinence (UI) is a disorder that in general affects 1 to 39% of men. It has various etiologic causes and the main one is UI secondary to radical prostatectomy (RP). Stress UI (SUI) secondary to RP continues to be a problem with a significant impact on patient quality of life. Treatment depends on whether the UI is mild, moderate, or severe. Severe UI requires more intense methods that simulate urethral sphincter activity. The options are the transobturator sling or artificial urinary sphincter implantation, accepted as the gold standard. A 68-year-old man had onset of lower urinary tract symptomatology in 2006. Study protocol reported a prostate-specific antigen (PSA) value of 7.02 ng/ml and a free PSA fraction of 17%. The histopathologic study (HPS) of the prostate biopsy specimens reported prostate cancer (CaP). RP was performed and the HPS reported a Gleason score of 4 + 4 = 8 and stage pT2c disease. The patient later presented with moderate SUI that did not improve with medical treatment. Cystourethrography revealed dilation of the bulbous urethra and cystoscopy showed an integral sphincter, so surgical management with transobturator sling (AdVance®) placement was decided upon. It is suggested that in the majority of cases all of the following should be carried out prior to surgery: a complete medical history, physical examination, laboratory studies, international UI scale questionnaire, cystoscopy, and urodynamics study. Sling placement is currently indicated in the management of mild to moderate UI and external artificial sphincter is indicated in moderate to severe cases. SUI management after RP continues to be a challenge for the urologist, despite the available therapeutic options. The urethral sling has become a useful and less expensive treatment option, with a lower complication rate compared with the artificial sphincter, especially in patients with UI that is not severe. * Corresponding author at: Calzada de Tlalpan N° 4800, Colonia Sección XVI, Delegación Tlalpan, C.P. 14080, México D.F., México. Telephone: 4000 3044 (S. Ahumada-Tamayo). 0185-4542 - see front matter © 2013. Revista Mexicana de Urología. Publicado por Elsevier México. Todos los derechos reservados. Management of post-radical prostatectomy male urinary incontinence with a transobturator sling (AdVance®) Palabras clave Incontinencia urinaria de esfuerzo; Posprostatectomía radical; Cabestrillo transobturador; México. 209 Manejo de la incontinencia urinaria masculina posprostatectomía radical con cabestrillo transobturador (AdVance®) Resumen La incontinencia urinaria (IU) es un padecimiento que en general afecta a los hombres en 1% a 39%; tiene varias causas etiológicas, siendo la principal secundaria a prostatectomía radical (PR). La IU de esfuerzo (IUE) secundaria a una PR, continúa siendo un problema con un impacto importante en la calidad de vida del paciente. El tratamiento para la IU depende si se trata de una clasificación leve, moderada o severa. En la IU severa se requiere métodos más intensos, que semejen la actividad del esfínter uretral. Las opciones son el cabestrillo transobturador o implantación de esfínter urinario artificial, aceptado como “gold standard”. Se presenta hombre de 68 años de edad, quien inició en el 2006 con sintomatología obstructiva urinaria baja, se realizó protocolo de estudio con antígeno prostático específico (APE) de 7.02 ng/mL, fracción libre 17%, se realizaron biopsias de próstata con reporte histopatológico (RHP) de cáncer de próstata (CaP); se efectuó PR. El RHP evidenció Gleason 4+4=8 y pT2c. Posteriormente cursa con IUE, moderada, sin mejoría al tratamiento médico. La uretrocistografía mostró dilatación de la uretra bulbar y en la cistoscopia se observó el esfínter íntegro, por lo que se decidió su manejo quirúrgico con la colocación de cabestrillo transobturador (AdVance®). Se sugiere que previo a la cirugía se debe realizar en la mayoría de los casos historia clínica completa, exploración física, estudios de laboratorio, cuestionario de la escala internacional de IU, cistoscopia y estudio de urodinamia. En la actualidad, la colocación del cabestrillo está indicada en el manejo de la IU de leve a moderada, y el esfínter artificial externo en el caso de moderada a severa. El manejo de la IU de esfuerzo posprostatectomía radical (PPR), continúa siendo un reto para el urólogo a pesar de las opciones terapéuticas que se tienen en estos momentos. El cabestrillo uretral se convierte en la actualidad en una opción útil de tratamiento menos costosa, con disminución de la tasa de complicaciones en comparación al esfínter artificial, sobre todo en los pacientes con IU no severa. Introduction Case presentation Urinary incontinence (UI) is a condition that generally affects 5% to 69% of women and 1% to 39% of men. The etiology in each sex varies and the most frequent causes in men are advanced age, lower urinary tract symptoms (LUTS) plus infections, functional and cognitive deterioration, neurologic disorders, and radical prostatectomy (RP) as the principal cause. Stress urinary incontinence (SUI) secondary to RP continues to be a problem that has a significant impact on patient quality of life. The rate of UI after 12 postoperative months is reported to be from 5% to 30%. SUI after RP is mainly due to intrinsic sphincterial deficiency and less often to detrusor instability or pure extrinsic sphincterial deficiency.1 Among the initial treatment options for low-grade UI that can be implemented are pelvic floor exercises, electric stimulation, or drug therapy. A possibility is the injection of bulking agents to thicken the urethra, as well as the artificial sling described by Kaufman in 1970; he was the first to report on and utilize synthetic material as a sling or balloon compression devices. In cases of severe UI more intense methods that simulate urethral sphincter activity are necessary. An option prior to the artificial sphincter is the transobturator sling (AdVance®). Its application is easier and it has satisfactory success results. And finally, when total continence is desired, the option accepted as the gold standard is the artificial urinary sphincter.1-3 A 68-year-old man presented with lower urinary tract symptoms in 2006. Evaluation protocol produced a prostate-specific antigen (PSA) of 7.02 ng/mL and a free PSA fraction of 17%. The histopathologic study (HPS) of the prostate biopsies reported prostate cancer. RP was performed and the HPS reported a Gleason score of 4 + 4 = 8 and stage pT2c disease. The patient later presented with moderate SUI that did not improve with medical treatment. Cystourethrography showed dilation of the bulbous urethra (figs. 1 and 2) and cystoscopy revealed an integral sphincter, leading to the decision of surgical management with the placing of a transobturator sling (AdVance®) (figs. 3 to 6). The postoperative progression was adequate. Discussion We know that in the case of post-radical prostatectomy (PRP) SUI, the external artificial sphincter is the gold standard and has a continence rate of 73% to 90%. Nevertheless, there have been important complications and re-interventions up to 57% in the follow-up of these patients. Among the complications are erosions, infections, UI, and mechanical problems of the sphincter requiring re-intervention, all of which imply greater cost to the patient and surgical procedures for non-physiological urinary emptying. Therefore it is necessary to contemplate a less invasive alternative to the external artificial sphincter, such as the transobturator 210 S. Ahumada-Tamayo et al Figure 1 Voiding cystourethrogram in the elimination phase: the bulbous urethra can be seen. Figure 2 Voiding cystourethrogram: a greater opening of the bulbomembranous urethra can be seen. Figure 3 The lithotomy position with the areas for approach marked on the skin in the perineum and under the tendon of the adductor magnus muscle. Figure 4 Insertion of the helical needle from the obturator fossa to the perineum. Figure 5 The sling with the 2 ends of the external mesh can be seen in the bulbous urethra position. Figure 6 Visualization of the bulbous urethra with the mesh now in place and the floor of the urethra elevated. sling.3 The first pioneers to carry out these types of transobturator sling procedures in which the posterior urethra has a more proximal relocation and thus better continence were Rehder and Gozzi.3,4 They conducted studies on cadavers and then on male patients and they had an incontinence cure rate of 40%, an incontinence improvement rate of 30%, and a minimal morbidity rate.4 It is suggested that prior to surgery in the majority of cases a complete anamnesis, physical examination, laboratory studies, international urinary incontinence scale questionnaire, cystoscopy, and urodynamics study should be carried out. Currently, transobturator sling placement is indicated in the management of mild to moderate UI and external artificial sphincter is indicated in moderate to severe UI.5-7 An important point to consider is that in case of sling failure, external artificial sphincter placement can be offered.1,4,8 Conclusions The management of PRP SUI continues to be a challenge for the urologist despite the current therapeutic options. A determining factor for treatment success is the experience of the surgeon in managing the different treatment modalities. The urethral sling has presently become a useful and less expensive treatment option with a lower complication rate compared with the artificial sphincter, especially in patients that do not present with severe UI. Conflict of interest The authors declare that there is no conflict of interest. Financial disclosure No financial support was received in relation to this article. References 1. Hubert J. Bulbourethral composite suspension: a new operative technique for post-prostatectomy incontinence. J Urol 2004;171(5):1866-1870. 2. Thüroff JW, Abrams P, Andersson KE, et al. Guía clínica sobre la incontinencia urinaria. Actas Urol Esp 2011;35(7):373-388. 3. Cornel EB, Elzevier HW, Putter H. Can Advance Transobturator Sling Suspension Cure Male Urinary Postoperative Stress Incontinence? J Urol 2010;183(4):1459-1463. Management of post-radical prostatectomy male urinary incontinence with a transobturator sling (AdVance®) 4. Rehder P, Gozzi C. Transobturator Sling Suspension for Male Urinary Incontinence Including Post-Radical Prostatectomy. Eur Urol 2007;52(3):860-866. 5. Castle EP, Andrews PE, Itano N, et al. The male sling for postprostatectomy incontinence: mean followup of 18 months. J Urol 2005;173(5):1657-1660. 211 6. Inci K, Ergen A, Bilen CY, et al. A new device for the treatment of post-prostatectomy incontinence: adjustable perineal male sling. J Urol 2008;179(2):605-609. 7. Han JS, Brucker BM, Demirtas A, et al. Treatment of Post-Prostatectomy Incontinence With Male Slings in Patients With Impaired Detrusor Contractility on Urodynamics and/or Who Perform Valsalva Voiding. J Urol 2011;186(4):1370-1375. Rev Mex Urol 2013;73(4):212-215 ÓRGANO OFICIAL DE DIFUSIÓN DE LA SOCIEDAD MEXICANA DE UROLOGÍA www.elsevier.es/uromx Clinical case Management of recurrent stricture of the perineal meatus with the Blandy technique after penectomy secondary to corpora cavernosa abscess J. Á. Martíneza,*, E. A. Ramírez-Pérezb, A. J. Camacho-Castroa, V. Osornio-Sáncheza, S. Ahumada-Tamayoa, G. Fernández-Noyolaa, F. J. García-Salcidoa, E. L. Muñoz-Ibarraa, E. Mayorga-Gómeza, G. Garza-Sainza, M. Cantellano-Orozcoa, J. G. Morales-Montora, C. Martínez-Arroyoa and C. Pacheco-Gahblera a Department of Urology, Hospital General “Dr. Manuel Gea González”, Mexico City, Mexico b Centro de Uretra México, Mexico City, Mexico KEYWORDS Perineal urethrostomy; Perineal meatus; Blandy technique; Mexico. Management of recurrent stricture of the perineal meatus with the Blandy technique after penectomy secondary to corpora cavernosa abscess Abstract Perineal urethrostomy stricture is a frequent complication and its management is difficult to treat. The aim of this article was to describe the specific aspects related to the Blandy technique. A 62-year-old man had a past medical history of drainage of an idiopathic abscess of the corpora cavernosa. One year later he presented with necrosis of the glans penis and the corpora cavernosa and underwent partial penectomy. He then presented with complex stricture of the urethra that was resolved with first-stage Johanson repair. The patient was seen at the Plastic and Reconstructive Surgery Service and underwent resection of the penile remnant and the formation of a neophallus with a radial forearm free flap. He presented with necrosis and flap loss. Total penectomy and perineal urethrostomy were performed. One year later the patient presented with a decrease in urinary stream caliber and acute urinary retention that was resolved through cystostomy and perineal urethrostomy reconstruction. He presented with re-stricture that was treated with urethral dilations. The gradual diminishing of the urinary stream caliber persisted and so a perineal meatoplasty with the Blandy technique was performed. The surgical correction of perineal urethrostomy stricture with the Blandy technique is a valid and effective option in the treatment of this complication. * Corresponding author at: Calzada de Tlalpan N° 4800, Colonia Sección XVI, Delegación Tlalpan, C.P. 14080, México D.F., México. Telephone: 4000 3044. Email: doctorangel25@hotmail.com (J. Á. Martínez). 0185-4542 - see front matter © 2013. Revista Mexicana de Urología. Publicado por Elsevier México. Todos los derechos reservados. Blandy technique for the management of recurrent stricture of the perineal meatu Palabras clave Uretrostomía perineal; Meato perineal; Técnica de Blandy; México. 213 Resumen La estenosis de la uretrostomía perineal es una complicación frecuente, el manejo de la misma es un problema de difícil tratamiento. El objetivo del presente artículo es demostrar los aspectos específicos relacionados a la técnica de Blandy. Se presenta hombre de 62 años de edad, con antecedente de drenaje de absceso idiopático de cuerpos cavernosos. Un año despues presentó necrosis de glande y cuerpos cavernosos, por lo cual fue sometido a falectomía parcial. Presentó estenosis compleja de uretra, que se resolvió con primer tiempo de Johanson. Acudió al Servicio de Cirugía Plástica y Reconstructiva donde fue sometido a resección de remanente peniano y formación de neofalo con colgajo radial libre, presentando necrosis y pérdida del colgajo. Se realizó falectomía total y formación de uretrostomía perineal. Un año despues presentó disminución del calibre del chorro urinario y retención aguda urinaria, que se resolvió con cistostomía y plastía de uretrostomía perineal. Presentó reestenosis tratada con dilataciones uretrales, persistiendo disminución gradual del calibre del chorro urinario, por lo que se practicó plastía del meato perineal con técnica de Blandy. La corrección quirúrgica de la estenosis de la uretrostomía perineal con técnica de Blandy, es una opción válida y efectiva en el tratamiento de esta complicación. Introduction Perineal urethrostomy is a widely accepted option for the management of complex stricture of the anterior urethra and is most often used as the first stage in the reconstruction by stages or when total urethral restoration is not feasible.1 Stricture at the site of the perineal urethrostomy presents with a certain frequency in patients requiring this type of urethral reconstruction and its management is often a problem that is difficult to treat. Published information on the surgical correction of this complication is limited.2 Barbagli et al. recently published their 30-year experience with the posterior flap technique in perineal urethrostomy, which can also be used for the correction of perineal meatus stricture.3 This technique, originally described by Blandy as the hybridization of the principles established by TurnerWarwick, requires that the surgeon first determine the necessary length of the flap before making the incision in the skin. This procedure is especially difficult in obese patients or in those patients that present with abnormal perineal anatomy.4 The Turner-Warwick technique was modified by Blandy because he found this procedure to be extremely difficult, with problems of stricture recurrence of the flap’s vertex near the verumontanum. In 1968, Blandy described the inverted U-shaped scrotal flap urethroplasty, which was much easier to perform and did not appear to result in recurrent stricture. Some time later, Blandy again described how he had developed this surgical technique, believing it to be an original one, but then he realized that it had already been described by Leadbetter, Gil-Vernet, Wells, and Williams.4 Over the years, the two-stage procedures have been widely modified. In 1984 Schreiter described a new technique in stages using a skin graft5 and Venn and Mundy introduced the buccal mucosa graft in procedures by stages for patients with lichen sclerosus.6 In the era of single-stage procedure repair, there were still indications for urethroplasty in stages. The strictures associated with adverse local conditions such as fistula, false pathway, abscess, cancer, or a previous urethroplasty due to complex stricture, are better treated with procedures in stages. Perineal urethroplasty can be a temporary or a permanent solution to a complex penile, bulbar, or posterior urethral stricture. Some patients choose not to undergo any type of second or third-stage reconstruction and prefer to continue carrying out micturition through the perineal urethroplasty, and thus turning the first-stage procedure into the only one that is performed.7 The aim of this article was to demonstrate the technical aspects of the surgical management of perineal urethrostomy stricture following penectomy. Case presentation A 62-year-old Catholic man born and living in Mexico City, divorced, and retired had a past medical history of abscess of the corpora cavernosa that spontaneously originated in July 2006. He underwent exploratory surgery, abscess drainage, and partial penectomy. He later presented with complex urethral stricture that was resolved with first-stage Johanson repair. He spontaneously sought medical attention at the Plastic and Reconstructive Surgery Department with the intention of having penile reconstruction. He underwent stump resection and the formation of a neophallus with radial forearm flap. He presented with necrosis of the radial forearm flap and therefore underwent flap resection, total penectomy, and perineal urethrostomy formation. One year later he presented with a weakening of the urinary stream caliber through the perineal urethrostomy and so had urethral dilations with a urethral dilating balloon. He presented with acute urinary retention that was resolved through percutaneous cystostomy and later perineal urethrostomy reconstruction in December of 2009. One year after the perineal urethrostomy reconstruction he presented with recurrent stricture for which urethral dilations were begun, but there was a gradual decrease in the caliber 214 J. Á. Martínez et al A Figure 1 Identification of the perineal meatus and marking of the surgical site. B Figure 2 A) Incision over the previously marked lines and B) perineal incision outline. Figure 3 Dissection of the urethra and identification of the posterior urethra with the rhinoscope. of the urinary stream despite the dilations. Due to the failure of the perineal urethrostomy with balloon dilations, a new urethral meatoplasty was performed. On this occasion it was carried out with the Blandy technique, sparing the posterior urethral plate in order to reduce the risk for devascularization of the urethral remnant and in turn, providing a lower possibility of recurrent stricture. Description of the surgical technique Under peridural block, the patient was placed in the lithotomy position and antisepsis of the perineal region was done. The perineal meatus was cannulated with a 6Fr ureteral stent and instilled with gentian violet dye to pigment the urethral mucosa (fig. 1). A perineal incision in the shape of an inverted U was made (fig. 2). A posterior flap was formed with sufficient fatty tissue to preserve adequate tissue irrigation and the urethra was completely dissected keeping the posterior urethral plate intact. An automatic Scott separator was placed for adequate exposure of the anatomy of the region to be operated on. The urethra was spatulated with a 6 o’clock cut and the urethral lumen was inspected with a rhinoscope for verumontanum visualization (fig. 3). Figure 4 Perineal cutaneous flap plication. Absorbable 4-0 suture with a modified needle was used to move the skin of the flap up to the spongy tissue found immediately in front of the verumontanum. Three sutures were placed in this position; they were adjusted and in this way moved the edge of the inverted U-shaped perineal skin flap toward the edge of the urethral mucosa (fig. 4). The margins of the perineal skin were sutured to the margin of the bulbous urethral plate and a 20Fr silicon transurethral catheter was placed. A capillary drain was placed, which is normally removed on the third to fifth postoperative day, prior to the patient’s release. The procedure was performed with no complications. The patient was released on the second postoperative day and the transurethral catheter was removed on postoperative day 21. He currently presents with adequate urine flow and no signs of stricture (fig. 5). Postoperative surveillance is carried out at 3, 6, and 9 months and then yearly. All patients undergo uroflowmetry and a physical examination with rhinoscope through the perineal meatus in order to evaluate adequate stoma permeability. The primary results analyzed are treatment success or failure, which are defined as no evidence of stricture recurrence and evidence of stricture recurrence, respectively.4 Blandy technique for the management of recurrent stricture of the perineal meatu A 215 B Figure 5 A) Layer closure. B) Appearance of the final results. Discussion Conflict of interest The success rate of perineal urethrostomy success rate according to the etiology of the stricture has been shown to be high in patients with failed hypospadias repair (87.5%), compared with patients with stricture secondary to infectious processes (33.3%). It is likely that any type of stricture loses its identity with time and all the strictures, regardless of their etiology, become an identical pathologic process due to the repeated treatments (dilations, urethrotomy, urethroplasty), making it appear that the original etiology of the stricture does not influence the final result.8 The lack of a tool for evaluating the result of urethral reconstructive surgery should motivate us elaborate questionnaires that can measure the most important aspects of the health status of a patient that has undergone perineal urethrostomy. In the future, it will be obligatory to develop questionnaires that specifically deal with urethral pathology based on a clearly defined conceptual framework indicating the importance of the patient’s perspective and expectations.3 The authors declare that there is no conflict of interest. Conclusions Perineal urethrostomy stricture is a frequent complication whose treatment is complex due to the high rate of stricture recurrence that presents when the adequate technique is not performed. Surgical correction of a urethrostomy stricture with the Blandy technique is a valid and effective option in the treatment of this complication. Financial disclosure No financial support was received in relation to this article. References 1. French D, Hudak SJ, Morey AF. The “7-Flap” Perineal Urethrostomy. Urology 2011;77(6):1487-1489. 2. Blandy JP, Singh M, Tresidder GC. Urethroplasty by scrotal flap for long urethral strictures. Br J Urol 1968;40(3):261-267. 3. Barbagli G, De Angelis M, Romano G, et al. Clinical Outcome and Quality of Life Assessment in Patients Treated With Perineal Urethrostomy for Anterior Urethral Stricture Disease. J Urol 2009;182(2):548-557. 4. Blandy JP. One-stage and two-stage urethroplasty. In: Reconstructive Urologic Surgery; Pediatric and Adult. Baltimore: Williams & Wilkins; 1977. p. 275-286. 5. Schreiter F. Mesh-graft urethroplasty: our experience with a new procedure. Eur Urol 1984;10(5):338-344. 6. Venn SN, Mundy AR. Urethroplasty for balanitis xerotica obliterans. Br J Urol 1998;81(5):735-737. 7. Secrest CL. Staged urethroplasty: indications and techniques. Urol Clin North Am 2002;29(2):467-475. 8. Barbagli G, De Angelis M, Romano G, et al. Long-term followup of bulbar end-to-end anastomosis: a retrospective analysis of 153 patients in a single center experience. J Urol 2007;178(6):2470-2473. Rev Mex Urol 2013;73(4):216-219 ÓRGANO OFICIAL DE DIFUSIÓN DE LA SOCIEDAD MEXICANA DE UROLOGÍA www.elsevier.es/uromx Clinical case Technical aspects of laparoscopic partial nephrectomy S. Ahumada-Tamayo*, J. Á. Martínez, G. Fernández-Noyola, F. García-Salcido, E. MuñozIbarra, A. J. Camacho-Castro, E. Mayorga-Gómez, V. Osornio-Sánchez, G. Garza-Saenz, V. Cornejo-Dávila, A. Palmeros-Rodríguez, I. Uberetagoyena-Tello de Meneses, C. Martínez-Arroyo, M. Cantellano-Orozco, J. G. Morales-Montor and C. Pacheco-Gahbler Department of Urology, Hospital General “Dr. Manuel Gea González”, Mexico City, Mexico KEYWORDS Renal cell carcinoma; Laparoscopic partial nephrectomy; Open partial nephrectomy; Mexico. Abstract Renal cell carcinoma (RCC) represents 3% of all malignant tumors in the adult, with a man:woman ratio of 2:1. It is more frequent between the ages of 60 and 70 years, and 80% of the patients present with the histologic clear cell variant. Currently, 50% of patients are diagnosed incidentally. A 71-year-old man had illness onset in March 2012 with gross hematuria, developing symptoms of acute urine retention. A transurethral catheter (TUC) was placed and the hematuria remitted. Study protocol was carried out, and the urotomography (UroCAT) scan identified a heterogeneous tumor on the lateral surface of the upper pole of the right kidney that measured 45 x 40 mm and had a radiodensity of 20 HU with up to 120 HU enhancement, plus a simple left Bosniak 1 renal cyst. A right laparoscopic partial nephrectomy (LPN) was performed using the transperitoneal abdominal approach with dissection of the renal unit. Upon locating the renal mass, the renal hilum was clamped under warm ischemia. The tumor was resected, bovine thrombin (Floseal®) was placed at the resection site, and mattress sutures were used to suture the fatty tissue patch. The histopathologic study reported the eosinophilic variant of chromophobe carcinoma, pT1b NO MO. There has been a significant increase in nephron-sparing surgery (LPN) to date and its main usefulness has been in localized tumors. Depending on tumor location, the approaches are transperitoneal, retroperitoneal, and hand-assisted. LPN has the important benefit of being minimally invasive, maintaining the function of the rest of the renal parenchyma. LPN is an alternative to open partial nephrectomy (OPN) when performed by an experienced surgeon and on selected patients. The ideal indication for LPN is a small, peripheral renal tumor. * Corresponding author at: Calzada de Tlalpan N° 4800, Colonia Sección XVI, Delegación Tlalpan, C.P. 14080, México D.F., México. Telephone: 4000 3044. (S. Ahumada-Tamayo). 0185-4542 - see front matter © 2013. Revista Mexicana de Urología. Publicado por Elsevier México. Todos los derechos reservados. Technical aspects of laparoscopic partial nephrectomy Palabras clave Carcinoma de células renales; Nefrectomía parcial laparoscópica; Nefrectomía parcial abierta; México. 217 Nefrectomía parcial laparoscópica. Aspectos técnicos Resumen El carcinoma de células renales (CCR) representa 3% de todas las neoplasias malignas del adulto, con una relación hombre:mujer de 2:1; se presenta más frecuente entre los 60 y 70 años de edad, con una variante histológica de células claras en el 80%. Actualmente, el 50% se diagnostica en forma incidental. Se presenta hombre de 71 años de edad, inicia su padecimiento en marzo de 2012 con hematuria macroscópica, desarrollando cuadro de retención aguda de orina. Se colocó sonda transuretral (STU), remite la hematuria y se realiza protocolo de estudio, encontrando en la urotomografía (UROTAC) tumor renal en polo superior de riñón derecho, en la cara lateral, heterogéneo, de 45 x 40 mm, 20 UH, que refuerza hasta 120 UH, sumado a un quiste renal simple izquierdo Bosniak I. Se realiza nefrectomía parcial laparoscópica (NPL) derecha. Técnicamente: abordaje abdominal transperitoneal con disección de la unidad renal, al localizar la masa renal se realiza isquemia caliente en hilio renal, se reseca tumor, se coloca trombina bovina (Floseal®) en lecho de resección y parche de tejido graso, se dan puntos de colchonero. Reporte histopatológico: carcinoma cromófobo variante de células eosinófilas, pT1bN0M0. La cirugía conservadora de nefronas (NPL) ha tenido un aumento importante a la fecha, con principal utilidad en tumores localizados. Dentro de los aspectos técnicos existe el abordaje transperitoneal, retroperitoneal y mano asistida, dependiendo de la ubicación del tumor. La NPL tiene importancia en el beneficio de mínima invasión, manteniendo la función del resto del parénquima renal. En manos expertas y con pacientes seleccionados, la NPL es una alternativa a la nefrectomía parcial abierta (NPA). La indicación óptima de la NPL es un tumor renal pequeño y periférico. Introduction Renal cell carcinoma (RCC) represents 3% of all malignant tumors in the adult, with a man:woman ratio of 2:1. It is more frequent in the fourth and sixth decades of life and 80% of the cases are the clear cell histologic variant. Currently, 50% of the cases are diagnosed incidentally, the majority through imaging techniques such as ultrasonography and computerized tomography (CT). Partial nephrectomy has gained importance in cases of localized tumors, with the modalities of open partial nephrectomy (OPN) and laparoscopic partial nephrectomy (LPN).1-4 The latter, minimally invasive, surgery was introduced by McDougall and Winfield in 1993, and over the years has had greater reproducibility. Indications for LPN are: absolute (a single anatomic or functioning kidney), relative (an affected contralateral kidney with deteriorating kidney function), and optional (localized unilateral kidney cancer with a healthy contralateral kidney).5 Case presentation A 71-year-old man was diagnosed with high blood pressure in 1999 and is in treatment with metoprolol. He has a past medical history of TURP in 2001. His present disease onset was in March 2012 with gross hematuria, developing symptoms of acute urine retention (AUR). A transurethral catheter (TUC) was placed and the hematuria remitted. In the study protocol, a urotomography (UroCAT) scan revealed a heterogeneous renal tumor on the lateral surface of the upper pole of the right kidney that measured 45 x 40 mm and had a radiodensity of 20 HU with up to 120 HU enhancement, plus a simple left Bosniak I renal cyst (figs. 1 and 2). A right LPN through the transperitoneal abdominal approach was performed with dissection of the complete renal unit. When the renal mass was located, warm ischemia at the renal hilum was carried out. The tumor was resected, bovine thrombin (Floseal®) was placed at the resection site, along with gelfoam and a fatty tissue patch, and sutured with mattress sutures (figs. 3 to 6). The histopathologic report stated eosinophilic variant of chromophobe renal cell carcinoma with a 4.6 x 4.2 x 3.3 cm tumor with no lymphovascular infiltration and stage pT1bN0M0 disease. The patient is presently continuing his oncologic follow-up. Discussion There has been an important increase to date in nerve-sparing LPN surgery and its principal use is in localized T1a or T1b tumors. The transperitoneal, retroperitoneal, and hand assisted approaches are among its technical aspects. 1 A transperitoneal approach is simpler in anterior tumors that are located in the lower pole, than in those on the posterior or superior surface, which are ideal candidates for a retroperitoneal approach. However, total kidney dissection can make complete renal exposure possible in a transperitoneal approach. Hand-assisted surgery can be useful in concrete cases of large-volume tumors, enabling better control of hemorrhage with manual compression, thus prolonging the work time and therefore minimizing the warm ischemia period in cases of difficult access or large tumor volume.6,7 It is important to see the location of the tumor. In the case of the transperitoneal approach the patient is positioned at a 30⁰ angle and 4 to 5 trocars are placed. The kidney is dissected, mannitol is administered, proceeding to warm ischemia (a time not over 30 to 40 min is essential) and to the partial nephrectomy with a monopolar device. A 218 S. Ahumada-Tamayo et al Figure 1 Arterial phase of the urotomography scan: it shows a tumor on the upper pole of the right kidney and a Bosniak I simple cyst on the left kidney. Figure 2 Elimination phase of the urotomography scan: it shows a tumor on the right kidney and a simple cyst on the left kidney. Figure 3 Dissection of the right kidney and its hilum reference point. Figure 4 The tumor can be seen on the pole of the right kidney and part of the partial nephrectomy. Figure 5 Floseal® placement at the partial nephrectomy surgical site. Figure 6 Gelfoam placement at the right partial nephrectomy surgical site. cold-knife incision is made at the level of the renal medulla, bovine thrombin (Floseal®) is placed at the surgical site with Monocryl™ 3-0 mattress sutures, interposed with gelfoam. A drain is placed and the trocar area is closed.2,5 LPN is a complex technique, even for the experienced surgeon, and it has a high complication rate that includes intra and postoperative bleeding and urinary fistulas. Positive surgical margins are the most important complications.8,9 Although it is true that surgery duration is longer with LNP than with OPN and the postoperative complication rate is greater (kidney failure, urinary fistulas, blood loss) these factors will decrease in relation to the learning curve. The importance of this surgery is the benefit of minimal invasion, maintaining the function of the rest of the renal parenchyma.1,2,5 Conclusions In the hands of experienced surgeons and with selected patients, LPN is an alternative to OPN. The ideal indication for LPN is a small and peripheral tumor. Long-term kidney function is dependent on the length of time of the intraoperative ischemia. LPN has a higher complication rate than open surgery, but it is now known that the result in the oncologic follow-up is similar to that obtained with OPN. Conflict of interest The authors declare that there is no conflict of interest. Financial disclosure No financial support was received in relation to this article. References 1. Colombo JR Jr, Gill IS. Nefrectomía parcial laparoscópica: Técnica y resultados. Actas Urol Esp 2006;30(5):501-505. 2. Cáceres, Núnez Mora, Cabrera, García Mediero, García Tello, Angulo. Nefrectomía parcial laparoscópica, técnica quirúrgica. Actas Urol Esp 2011;35(8):487-493. 3. Tolosa Eizaguirre E, Pascual Piedrola J, Barba Abad J, et al. Nefrectomía parcial laparoscópica. Análisis de los primeros 30 casos de nuestra serie y revisión de la literatura. Actas Urol Esp 2010;34(9):798-801. 4. Kerbl DC, McDougall EM, Clayman RV, et al. A History and Evolution of Laparoscopic Nephrectomy. Perspectives from the Past and Future Directions in the Surgical Management of Renal Tumors. J Urol 2011;185(3):1150-1154. 5. Rosales Bordes A, Salvador Bayarri J, de Graeve N, et al. Nefrectomía parcial laparoscópica transperitoneal en el tratamiento del tumor renal. Actas Urol Esp 2006;30(5):492-500. Technical aspects of laparoscopic partial nephrectomy 6. Duke Herrell S, Laparoscopic partial nephrectomy techniques: Developments and translation. J Urol 2004;172(6 Pt 2):25532556. 7. Benway BM, Bhayani SB, Rogers CG, et al. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol 2009;182(3):866-872. 219 8. Permpongkosol S, Bagga HS, Romero FR, et al. Laparoscopic Versus Open Partial Nephrectomy for the Treatment of Pathological T1N0M0 Renal Cell Carcinoma: A 5-Year Survival Rate. J Urol 2006;176(5):1984-1988. 9. Winfield HN, Donovan JF, Godet AS, et al. Laparoscopic Partial Nephrectomy: Initial Case Report for Benign Disease. J Endourol 1993;7(6):521-526.