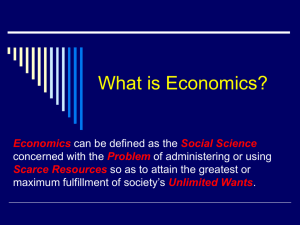
Importance of light and CO2 on the effects of endomycorrhizal
advertisement

New Phytol. (1999), 142, 539–550 Importance of light and CO on the effects # of endomycorrhizal colonization on growth and photosynthesis of potato plantlets (Solanum tuberosum) in an in vitro tripartite system D. L O U C H E-T E S S A N D I E R", G. S A M S O N#, C. H E R N A! N D E Z-S E B A S T I A' #†, P. C H A G V A R D I E F F" Y. D E S J A R D I N S#* " CEA-DEVM, LaP, Cadarache, 13108 Saint-Paul-lez-durance, France # Centre de Recheche en Horticulture, Pavillon de l’Envirotron, UniversiteT Laval, QC, Canada Received 7 September 1998 ; accepted 23 February 1999 A factorial analysis was conducted to investigate the effects of different levels of photosynthetic photon flux (PPF) and CO concentration on the interactions between the vesicular–arbuscular endomycorrhizal fungus Glomus # intraradices and potato plantlets (Solanum tuberosum) cultured in an in vitro tripartite system. We observed that CO enrichment from 350 to 10000 ppm stimulated root colonization by the fungus, and that this stimulation was # more pronounced under high PPF (300 µmol m−# s−") than low PPF (60 µmol m−# s−"). Consistent with these observations, the effects of G. intraradices on dry matter production in potato plantlets were strongly dependent on the CO and PPF levels during cultivation. There was no significant effect of the mycorrhizal fungus on dry # matter production at 350 ppm of CO . However, under the high CO concentration, mycorrhiza had opposite # # effects on dry matter production depending on the PPF : a decrease (k21%) and a stimulation (j25%) of dry matter production after 2 wk of growth under low and high PPF, respectively, were observed in presence of G. intraradices relative to plantlets grown in its absence. Furthermore, in mycorrhizal plantlets grown under high levels of both PPF and CO , the chlorophyll and carotenoid contents as well as the quantum yields of # photosynthetic electron transport and the photochemical quenching qP of the chlorophyll-a fluorescence measured near the PPF during growth were all higher than in non-infected plantlets. Our results therefore indicate that mycorrhizal G. intraradices can alleviate the down regulation of photosynthesis related to sink limitation, and its effect on dry matter production is strongly dependent on the levels of CO and PPF during growth which # determine the balance between the photosynthetic carbon uptake by the plantlets and the carbon cost by the fungus. Key words : chlorophyll-a fluorescence, CO enrichment, Glomus intraradices, micropropagation, mycorrhiza, O # # evolution. Symbiotic arbuscular mycorrhizal fungi can confer several benefits to their host plants such as, enhanced uptake of phosphorus (P) and other nutrients (Finlay et al., 1992 ; Pearson & Jackobson, 1993), resistance *Author for correspondence (tel 418 656 2131, extn 2359 ; e-mail Yves.Desjardins!plg.ulaval.ca). †Present address : Department of Chemistry & Biochemistry, New Mexico State University, Las Cruces, NM 88003, USA. to drought stress (Subramanian & Charest, 1995) and to pathogens (Perrin, 1990). In return, the mycorrhizal fungi obtain plant carbohydrates for their growth and maintenance ; c. 10% of carbon (C) translocated to the roots pass to the fungal partner (reviewed by Fitter, 1991). In order to improve plant growth, the benefits from a mycorrhizal association must be accompanied by a stimulation of photosynthetic C uptake that will at least compensate the C lost to the fungus. It is Printed from the C JO service for personal use only by... 540 D. Louche-Tessandier et al. generally assumed that enhancement of photosynthetic rates results from increased levels of leaf P as a result of the mycorrhizal contribution to the P plant uptake (Fitter, 1991). However, such stimulation of photosynthesis will depend on other environmental factors such as atmospheric CO and # incident light levels. For the host plant, these factors can therefore modify the balance between the costs, and the benefits, of a mycorrhizal relationship. In general, CO enrichment stimulates growth and # photosynthesis of C plants (Farrar & Williams, $ 1991). Whereas in the short term, CO enrichment # stimulates photosynthesis through the suppression of photorespiration (Gerbaud & Andre! , 1980), its long term effects depend on the equilibrium between production of carbohydrates in source leaves, their loading into and unloading from the phloem, and their utilization in sink organs (Farrar & Williams, 1991). In situations of insufficient sink strength relative to the C assimilation activities, sugars accumulate in source leaves and trigger a downregulation of photosynthesis that balances the source and sink activities (Bowes, 1991). It was suggested that mycorrhizal fungi can represent a significant sink for any excess of assimilates (Hodge, 1996 ; Wright et al., 1998) and therefore decrease the plant susceptibility to down-regulation of photosynthesis under prolonged exposure to elevated CO . Mycor# rhizas can further delay down-regulation of photosynthesis by increasing the uptake of nutrients required to sustain the stimulated plant growth and the formation of new sink organs (Lewis & Strain, 1996). There are several examples where CO enrichment # increases the percentage of ecto- or vesicular– arbuscular (VA)-mycorrhizal colonization in tree and grass species although this effect is somewhat variable (reviewed by Hodge, 1996 ; see also Rillig et al., 1998). Stimulation of mycorrhizal colonization under elevated CO concentrations may be caused by # an increase of C allocation to the root system therefore stimulating root growth, especially the fine roots which are the main site of mycorrhizal infection (Hodge, 1996). In in vitro culture systems, enhancement of mycorrhizal colonization may also be related to the stimulation of hyphal growth by synergistic effects between high CO concentrations # (0n5–1 %) and flavonoid compounds in root exudates (Chabot et al., 1992a ; Elmeskaoui et al., 1995). In both cases, an increase in the colonization rate will increase the total C cost of the mycorrhizal infection which was assumed to be directly proportional to the number of infection sites over the whole root system and to the total mycorrhizal respiration (Fitter, 1991). However in sink-limited plants growing under high CO , the relative cost of the C exported to the # mycorrhizal fungi may decrease despite enhanced colonization rates as photosynthesis is able to replenish its C pool under these conditions (Lewis & Strain, 1996). Therefore, the impacts of a mycorrhizal colonization on the balance between plant costs and benefits would be determined largely by the plant source–sink equilibrium. In this study, we investigated the interactive effects of CO concentrations (350 and 10 000 ppm) # and PPF (60 and 300 µmol m−# s−") on the relationship between the vesicular–arbuscular fungus Glomus intraradices and potato plantlets (Solanum tuberosum) cultured in an in vitro tripartite system as described by Elmeskaoui et al. (1995). Our objectives were to determine, under controlled conditions, how changes in the mycorrhizal colonization rate and of the plant source–sink relationship would alter the balance between the costs and the benefits for the mycorrhizal potato plantlets. We used a very high CO concentration # (1%) because of its stimulating effect on the mycorrhizal colonization rate in strawberry plantlets cultured in an in vitro tripartite system (Elmeskaoui et al., 1995). Also, the use of very high CO # concentrations is common in artificial growth conditions such as in vitro culture system (Gouk et al., 1979) and proposed life support systems in space (Reuveni & Bugbee, 1997). Plant material Potato plantlets (Solanum tuberosum L. cv. Lp 89–221 from the experimental farm of the Agriculture and Agri-food Canada at La Pocatie' re, QC, Canada) were multiplied in vitro and subcultured on MS solid medium (Murashige & Skoog, 1962) (0n55 mM myo-inositol, 27 µM glycine, 2n4 µM pyridoxine-HCl, 4n1 µM nicotinic acid, 1n2 µM thiamine-HCl and no growth regulators). Plantlets were kept in a growth chamber at a constant temperature of 23mC and illuminated for 16 h d−" under a PPF of 60 µmol m−# s−". One wk before mycorrhizal infection, nodal sections of potato vitroplants (apex and the four upper leaves) were excised and transferred on Sorbarod2 cellulose support (Baum Gartner Paper, Lausanne, Switzerland) imbibed with 3n5 ml of half-strength MS liquid medium containing 3% sucrose to induce rooting. Fungal inoculum The fungal inoculum used for the VA mycorrhiza in this study was G. intraradices Schenk & Smith (DAOM 197198, Biosystematic Research Center, Ottawa, Canada). Spores of G. intraradices were obtained from their co-culture with isolated tomato roots on minimal White medium according to Chabot et al. (1992a). For primary colonization (i.e. the production of G. intraradices inocula in root- Printed from the C JO service for personal use only by... Growth and photosynthesis of mycorrhizal potato plantlets organ cultures), new tomato roots (7 cm long) were placed in the presence of 200 viable fungal spores and incubated in darkness at 25mC for 10 wk in 15 cm polycarbonate petri dishes sealed with Parafilm. Since tomato root-organ cultures show a lower colonization rate than carrot root-organ cultures, 200 viable spores rather than 40 as in Elmeskaoui et al. (1995) were used in order to reduce the time required for the primary root colonization by the fungus. Then five healthy lateral roots with intact root apices were cut into 4-cm sections and placed in a Magenta2 (Magenta Corp., Chicago, IL, USA) polycarbonate box for 30 d. At the end of this period, the roots were 100% infected and hyphae were visible on the surface of the culture medium and the walls of the growth container. At this stage, the coculture was ready for the establishment of the secondary mycorrhiza. 541 (350 and 10 000 ppm), and PPFs (60 and 300 µmol m−# s−"). The experimental unit consisted of one Magenta box containing four vitroplants. For the two experimental blocks, eight Magenta pots, each containing either four control or four mycorrhizal potato vitroplants, were placed in 60 l growth chambers in which normal or CO -enriched # atmospheres were circulated at a flow rate ensuring a complete renewal within 30 min. Illumination was provided by a combination of incandescent and fluorescent lamps (60 µmol m−# s−") or by high pressure sodium lamps (300 µmol m−# s−"). We assumed that the spectral difference between the two types of lamps was insignificant compared with the difference in light intensities. The photoperiod was 16 : 8 hr (light : dark) and the temperatures were 23p1mC (day) and 19p1mC (night). Biomass determination Tripartite culture system The protocol followed for the tripartite culture system is described in detail in Elmeskaoui et al. (1995). Briefly, the MS medium was removed by suction from the Sorbarod plugs supporting the vitroplants, and thoroughly rinsed with distilled water and replaced by the minimal M medium (MgSO :7H O, 2n97 mM ; Ca(NO ) :4H O, 1n22 % # $# # mM ; KNO , 0n79 mM ; KCl, 0n87 mM ; KH PO , $ # % 35 µM ; NaFe-EDTA, 21n8 µM ; MnCl , 30n3 µM ; # ZnSO :7H 0, 9n2 µM ; H BO , 24n2 µM ; KI, 4n5 µM ; % # $ $ CuSO :5H O, 0n52 µM ; Na MoO :2H O, 0n01 µM ; % # # % # myo-inositol, 277n5 µM ; glycine, 40n0 µM ; nicotinic acid, 4n1 µM ; thiamine-HCl, 0n30 µM ; pyridoxineHCl, 0n47 µM ; sucrose, 1% ; Gel-gro, 0n25% (pH 5n5)). This procedure was used to remove the remaining sucrose in the paper plugs which might interfere with the establishment of the secondary mycorrhiza. The secondary colonization (i.e. the colonization of vitroplants with G. intraradices already formed on root-organ cultures) was then established under aseptic conditions by placing four vitroplants per Magenta container (type GA7) in contact with sterile (control) or colonized (treatment) tomato roots. The tripartite culture was conducted over 4 wk and 10 ml of fresh liquid medium M was supplied aseptically to each Magenta container weekly. Experimental design A factorial 2i2i2 experiment, with two randomized complete blocks independent in time, was designed to study the interactive effects of environmental factors (CO and PPF) and endo# mycorrhizal colonization by G. intraradices on growth and photosynthesis of in vitro potato plantlets. The factors were ; the in vitro mycorrhiza (control and G. intraradices), CO concentrations # After 14 and 28 d of triculture, one potato vitroplant per pot was randomly taken for fresh and dry biomass determination. The plantlets were harvested with their roots which were carefully removed under water from their cellulose supports. Fresh and dry weights of the shoots and roots were measured on an analytical balance, and used to determine the plant water content and the dry root : shoot ratio. Photosynthetic O evolution # Light saturation curves of CO -supported O evol# # ution were made during the last week of the experiment on leaf discs from the second and\or third leaves of potato vitroplants grown under the different environmental conditions already described. Photosynthetic O evolution was # measured at 23mC with a LD2 leaf-disc electrode system (Hansatech Instruments Ltd, King’s Lynn, Norfolk, UK) in the presence of saturating CO # concentrations (5 %) as described by Walker (1989). After a dark period of 20 min, leaves were illuminated by an array of red light emitting diodes (LH36 209 Ultrabright) at a PPF of 240 µmol m−# s−" until complete induction of photosynthesis (measured as a stable O evolution rate) was # achieved. Then, rates of O evolution were measured # after 5 min illumination at each PPF, increasing from 20 to 1000 µmol m−# s−". The maximum quantum yield of O evolution (Φmax) was estimated # from the linear portion of the light saturation curves (0–100 µmol m−# s−") and the maximum capacity of photosynthesis for O evolution (Pmax) was measured # at saturating light and CO levels. # Chlorophyll-a fluorescence Simultaneously with O evolution, Chla fluorescence # was measured from leaf discs enclosed in the LD2 Printed from the C JO service for personal use only by... 542 D. Louche-Tessandier et al. leaf-disc electrode system with a PAM 101\103 chlorophyll fluorometer (Walz, Effeltrich, Germany). After at least 20 min of dark adaptation, the minimal level of Chla fluorescence (Fo) was measured with the non-actinic 1n6 kHz modulated light and the maximal Chla fluorescence level (Fm) was induced by a 1 sec saturating flash provided by a KL1500 Schott light source (Schott, Mainz, Germany). During the saturating flash, the frequency of the modulated measuring light was 100 kHz. After 5 min of illumination at each PPF, the steady state (Fs) and the maximal (Fmh) fluorescence levels were measured in a similar way to Fo and Fm. Immediately after the saturating flash, the actinic light was turned off and the minimal fluorescence level (Foh) was measured in presence of a far red light using a RG715 long-pass filter. From the different fluorescence levels, the maximum (Fv\Fm) (Adams et al., 1990) and the operational (∆F\Fmh) (Genty et al., 1989) quantum yield of PSII electron transport were determined and the coefficients of photochemical (qP) and non-photochemical quenching (qN) of chlorophyll-a fluorescence were calculated according to van Kooten & Snel (1990). The qP coefficient is an indication of the fraction of PSII reaction centres which are in an open state, where the oxidized primary quinone electron acceptor (QA) can accept, via a pheophytin molecule, an electron provided from the special Chla molecule P ')! (Krause & Weis, 1991). Complementary information is given by the qN coefficient, which indicates the fraction of absorbed light energy that is dissipated as heat in competition to the photochemical reactions and Chla fluorescence emission. Leaf-pigment contents and stomatal conductance The Chla and b contents as well as total carotenoids were measured on the second and third leaves, as for the O and fluorescence measurements. Leaves were # ground in aqueous acetone (80%) and the pigment concentrations were estimated from absorbance measurements of the extracts made at 470, 647 and 663 nm, according to Lichtenthaler & Wellburn (1983). Stomatal conductance was measured on Day 28 of the experiments on the abaxial side of the leaves with a diffusion porometer (AP4, Delta-T Devices, Cambridge, UK). Assessment of arbuscular-mycorrhizal colonization The roots of plants used for photosynthetic measurements were collected after 28 d of triculture and stored until staining in a 13 :5 :200 (v\v\v) solution of formaldehyde (37%) : glacial acetic (99n8%) : ethanol (50%). Before staining, roots were bleached in a boiling solution of 10% KOH for 10 min, rinsed with distilled water, neutralized with 1% HCl and then rinsed again with distilled water. Roots were stained in a lactoglycerol solution (50% lactic acid : water : glycerol, 2 : 1 : 1 w\w\w) containing tryphan blue 0n05% and heated until boiling. They were thereafter stored in a lactoglycerol solution (lactic acid (87%), glycerol (6%) and water (6%)). For root colonization assessment, countings were made according to the grid-line intersect method (Giovanetti & Mosse, 1980), using ai40 (model BHZ-RFL-T3, Olympus Optical Inc., Japan) magnification stereo microscope. Indistinct blue spots on roots were screened ati100 magnification. Statistical analysis Analysis of variance (ANOVA) were performed on the variables using the SuperANOVA statistical program (Abacus Concepts Inc., Berkeley, CA, USA) according to the General Linear Model. Before ANOVA, the homogeneity of the variance was checked using the Levine’s test and the residual graphic analysis. When required, the means were transformed to log(xj1) (Steel & Torrie, 1980). Percentage of mycorrhizal colonization In potato vitroplants grown in presence of the fungal inoculum in the tripartite culture system, the percentage values of root segments showing the presence of the VA-mycorrhizal G. intraradices (hyphae, arbuscules or vesicles) after 28 d of triculture varied from 4n4p0n6% to 7n8p1n0% depending on the growth conditions (Table 1). ANOVA of the means indicated that CO -enrich# ment significantly stimulated the colonization rate (P l 0n006). Growth characteristics The dry matter (DM) of potato plantlets grown for 14 and 28 d under the different experimental conditions are presented in Table 1. After 14 d of triculture, there was a significant interaction (Pl0n001) between the three factors CO , PPF and # mycorrhiza. In all treatments, DM production strongly increased with increasing PPF from 60 to 30 µmol m−# s−". CO -enrichment also stimulated DM, # except in mycorrhizal plantlets grown at low PPF where no difference was observed. However, the effect of VA mycorrhiza on DM production in potato vitroplants was highly dependent on the CO and # PPF levels during the triculture. Under normal CO # concentration, the mycorrhizal fungus did not affect DM production. However, under a CO -enriched # atmosphere, mycorrhizal effects on DM production depended on the incident PPF : a decrease (k21%) and a stimulation (j25%) of DM production after 2 Printed from the C JO service for personal use only by... Growth and photosynthesis of mycorrhizal potato plantlets 543 Table 1. Effects of inoculation with Glomus intraradices and of different levels of CO and photosynthetic # photon flux (PPF) on the percentage of roots showing the presence of mycorrhiza, on the production of dry matter (DM ) and on the root to shoot (R : S) ratio of potato plantlets cultured in an in vitro tripartite system CO # (ppm) 350 PPF (µmol m−# s−") 60 300 10 000 60 300 Significance (P values) Factors CO # PPF Mycorrhiza CO iPPF # CO iMycorrhiza # PPFiMycorrhiza CO iPPFiMycorrhiza # Error df Mycorrhiza 28 d NM M NM M NM M NM M % of infection 0 4n4p0n6 0 4n8p0n6 0 5n7p0n7 0 7n8p1n0 0n006 0n079 0n0001 0n259 0n006 0n079 0n259 39 DM (mg) 14 d 22n1p4n1 24n0p7n5 58n7p3n3 51n4p8n4 31n6p11 25n0p8n1 90n5p23 113p15 0n001 0n001 0n334 0n001 0n066 0n077 0n001 52 DM (mg) 28 d 42n9p6n1 43n9p9n8 91n8p36 90n6p18 61n4p9n4 43n9p14 122p19 136p36 0n0003 0n0001 0n830 0n020 0n884 0n261 0n169 40 R : S ratio 0n10p0n01 0n11p0n01 0n44p0n07 0n33p0n03 0n10p0n01 0n13p0n02 0n62p0n11 0n84p0n14 0n005 0n0001 0n493 0n011 0n245 0n496 0n390 16 MeanspSE. M, mycorrhizal ; NM, non-mycorrhizal. wk of growth under low and high PPF, respectively, were observed in mycorrhizal plantlets compared with control plantlets. The stimulatory effects of CO enrichment and # increased PPF on DM production (P 0n001) were also observed after 28 d (Table 1). Whereas the CO iPPFimycorrhiza interaction observed at 14 # d was no longer significant (Pl0n169) at 28 d (despite a 29% decrease and 11% increase in DM related to mycorrhiza at low and high PPF, respectively, under elevated CO ), there was still a # significant interaction between CO and PPF # (Pl0n020). The lack of significance of the CO iPPFimycorrhiza interaction at 28 d could # indicate a possible restriction of plant growth towards the end of the experiment because of insufficient space in the Magenta containers. After pooling plants grown in the presence and absence of the fungal inoculum, examination of the mean values of plant DM indicates that in the second half of the experiment (14–28 d of culture), the production of plant DM at low PPF was 88% of the DM at 14 d for both normal and high CO concentrations. However # at high PPF, this relative increase of DM was 66% under normal CO and only 26% under high CO # # concentrations. Clearly, the stimulatory effects of high CO and PPF on growth of potato plantlets # could not be sustained during the second part of the experiment. In addition to their effects on plant DM, the growth conditions strongly altered C allocation as seen by the changes of the root : shoot (R : S) ratio in potato plantlets (Table 1). ANOVA indicated a significant interaction of CO and PPF on the R : S # ratio (Pl0n0001). Increases of this ratio as PPF increased (from 60 to 300 µmol m−# s−") were observed at both CO concentrations whereas the # stimulating effect of elevated CO on the R : S ratio # was observed only at high PPF and not at low PPF. The R : S ratio was not significantly modified by the presence of mycorrhiza (Pl0n453). Water content and stomatal conductance The mean values of the water content and stomatal conductance of the potato plantlets after 28 d of growth under the different experimental conditions are presented in Table 2. The PPF was the main factor affecting plant-water content ; this was consistently lower under high PPF than low PPF. However the magnitude of the PPF effect was modulated by the CO concentration and the # presence of mycorrhiza, as indicated by the significant triple interaction (Pl0n0003) between the three factors. The lowest water-content value was found in the mycorrhizal plants grown under high PPF and CO . # A significant interaction (Pl0n009) between the three factors on stomatal conductance measured on day 28 was observed (Table 2). The major factor appeared to be the mycorrhiza ; the mycorrhizal plantlets had higher stomatal conductances than control plantlets, except in those grown under high PPF and CO . In these plants, the low stomatal # conductance indicates that the stomata were mostly closed at the time of the measurement. Printed from the C JO service for personal use only by... 544 D. Louche-Tessandier et al. Table 2. Effects of inoculation with Glomus intraradices and of different levels of CO and photosynthetic # photon flux (PPF ) on the plant-water content and on stomatal conductance of potato plantlets cultured for 28 d in an in vitro tripartite system CO # (ppm) PPF (µmol m−# s−") 350 60 300 10 000 60 300 Mycorrhiza Plant water content (%) Stomatal conductance (mm s−") NM M NM M NM M NM M 92n6p0n5 92n8p0n5 84n2p0n9 87n1p0n8 91n4p1n1 93n7p0n6 84n5p1n1 80n5p1n4 23n9p2n5 27n7p2n1 22n6p3n5 30n6p1n0 25n4p1n3 35n1p1n3 2n7p0n4 2n1p0n2 0n006 0n0001 0n219 0n015 0n041 0n420 0n0003 40 0n0001 0n0001 0n0012 0n0001 0n363 0n276 0n009 35 Significance (P values) Factors CO # PPF Mycorrhiza CO iPPF # CO iMycorrhiza # PPFiMycorrhiza CO iPPFiMycorrhiza # Error df MeanspSE. M, mycorrhizal ; NM, non-mycorrhizal. Table 3. Effects of inoculation with Glomus intraradices and of different levels of CO and photosynthetic # photon flux (PPF ) on the contents of Chl a, Chl b, Chlajb, and total carotenoids measured in leaves of potato plantlets after 28 d of culture in an in vitro tripartite system CO # (ppm) 350 PPF ( µmol m−# s−") 60 300 10 000 60 300 Significance (P values) Factors CO # PPF Mycorrhiza CO iPPF # CO iMycorrhiza # PPFiMycorrhiza CO iPPFiMycorrhiza # Error df Mycorrhiza NM M NM M NM M NM M Chl a (mg g−" f. wt) Chl b (mg g−" f. wt) Chlajb (mg g−" f. wt) Carotenoids (mg g−" f. wt) 2n45p0n25 2n41p0n30 1n38p0n17 1n46p0n08 2n26p0n27 2n67p0n28 0n32p0n06 0n55p0n18 0n85p0n10 0n92p0n11 0n50p0n05 0n48p0n03 0n92p0n10 1n02p0n11 0n15p0n03 0n15p0n03 3n26p0n82 3n32p0n99 1n88p0n56 1n94p0n27 3n18p0n89 3n69p0n95 0n47p0n23 0n84p0n63 0n54p0n06 0n59p0n07 0n36p0n04 0n37p0n03 0n56p0n06 0n64p0n06 0n18p0n02 0n26p0n07 0n009 0n0001 0n436 0n005 0n212 0n701 0n855 39 Pigment contents and photosynthetic activities Pigment contents in the potato vitroplants grown under the different experimental conditions are shown in Table 3. There were marked decreases of Chla, Chlb and total carotenoid contents in plantlets grown under high PPF compared with those grown at low PPF. These decreases were more pronounced in plantlets grown under both high PPF and CO , as # 0n003 0n0001 0n098 0n0001 0n214 0n733 0n300 39 0n0001 0n0001 0n122 0n0001 0n237 0n718 0n861 39 0n031 0n0001 0n082 0n0032 0n441 0n986 0n678 39 indicated by the significant interaction PPFiCO # between these two factors (P0n005). In leaves developed under high PPF and CO , leaf chlorosis # and in some cases leaf necrosis started to appear towards the end of the experiments. Although the PPF and CO treatments did not interact signifi# cantly with the mycorrhiza treatment, it is noteworthy that leaves from mycorrhizal plantlets grown under high PPF and CO maintained higher Chlajb # Printed from the C JO service for personal use only by... Growth and photosynthesis of mycorrhizal potato plantlets 25 (a) (b) (c) (d) 545 20 Rates of O2 evolution (µmole O2 m–2 s–1) 15 10 5 0 25 20 15 10 5 0 0 200 400 600 800 1000 0 200 400 600 800 1000 PPF (µmol m–2 s–1) Fig. 1. Light saturation curves of oxygen evolution from leaves of potato plantlets cultured in the absence (open circles) or presence (closed circles) of the mycorrhizal fungus Glomus intraradices under a photosynthetic photon flux (PPF) of 60 (a, b) or 300 (c, d) µmol m−# s−" and under CO concentrations of 350 (a, c) or 10 000 # (b, d) ppm. and total carotenoid contents (78% and 44%, respectively) compared with those of nonmycorrhizal plantlets grown under the same conditions. Light saturation curves of photosynthetic O # evolution were measured at saturating CO con# centration on the upper leaves of potato vitroplants grown under the different conditions (Fig. 1). For each curve, we determined the initial slope as an estimation of the maximum quantum efficiency of O # evolution (Φmax) and also the light-saturated rate of O evolution (Pmax) which represents the maximum # photosynthetic capacity (Walker, 1989). The results for the different treatments are shown in Table 4. As observed for the pigment contents, there are significant interactive effects of PPF and CO (P0n033) # and on the Pmax and the Φmax parameters. In leaves developed under normal CO concentration, Pmax # markedly increased with increasing PPF. The opposite effect was found in leaves grown under high CO , where an increase of PPF lead to a large # decrease of Pmax. No differences between the Pmax values could be detected between control and mycorrhizal plantlets. Similar values for the maximum photochemical yield of O evolution (Φmax) were obtained for the # different treatments, except for both control and mycorrhizal plantlets grown under high CO and # PPF where significant decreases were observed (Table 4). These results are supported by the Chla fluorescence Fv\Fm ratio measured after dark adaptation of the leaves. Here again, leaves developed under high CO and PPF had lower Fv\Fm # ratios than leaves from other treatments. The Chla fluorescence Fv\Fm ratio represent a good estimation of the maximum photochemical yield of PS II, which has been shown to be closely correlated to the maximum quantum efficiency of photosynthesis measured under limiting PPF (Adams et al., 1990). As observed with the photosynthetic pigments, the negative effect of high PPF and CO during growth # on Fv\Fm was lower in leaves from mycorrhizal plantlets than those from non-mycorrhizal plantlets grown under the same conditions. Further information on the effects of mycorrhizas and environmental conditions on the photosynthetic efficiencies of potato plantlets were obtained by the Chla fluorescence quenching analysis (Schreiber et al., 1995). In potato plantlets cultivated under normal CO , increase of PPF during the measure# ments caused a larger decrease of the effective photochemical yield of PSII electron transport Printed from the C JO service for personal use only by... D. Louche-Tessandier et al. 546 Table 4. Effects of inoculation with Glomus intraradices and of different levels of CO and photosynthetic # photon flux (PPF ) on the maximum quantum efficiency of O evolution (Φmax), on the maximum quantum # efficiency of photosystem II photochemistry (Fv\Fm) and on the maximum capacity of O evolution (Pmax) # measured in leaves of potato plantlets after 21–28 d of culture in an in vitro tripartite system CO # (ppm) 350 PPF ( µmol m−# s−") Mycorrhiza 60 NM M NM N NM M NM M 300 10 000 60 300 φ max ( µmol O µmol−" photons) # 0n073p0n008 0n073p0n003 0n073p0n003 0n066p0n001 0n063p0n006 0n068p0n007 0n037p0n003 0n044p0n014 Significance (P values) Factors CO # PPF Mycorrhiza CO iPPF # CO iMycorrhiza # PPFiMycorrhiza CO iPPFiMycorrhiza # Error df Pmax ( µmol O m−# s−") # Fv\Fm 0n758p0n005 0n767p0n003 0n725p0n005 0n750p0n014 0n763p0n008 0n761p0n002 0n514p0n054 0n579p0n111 Φ Fv\Fm 0n007 0n001 0n459 0n007 0n805 0n520 0n667 16 max 0n002 0n009 0n770 0n033 0n386 0n713 0n557 16 15n8p1n2 14n4p0n5 25n0p2n3 24n8p0n9 13n4p0n5 14n6p1n5 9n8p1n6 9n8p0n6 Pmax 0n0001 0n0418 0n936 0n0001 0n592 0n991 0n663 16 MeanpSE. M, mycorrhizal ; NM, non-mycorrhizal. 0·8 (a) (b) (c) (d) Quantum yield of PSII photochemistry (∆F/FM′) 0·6 0·4 0·2 0·0 0·8 0·6 0·4 0·2 0·0 0 200 400 600 800 1000 0 200 400 600 800 1000 PPF (µmol m–2 s–1) Fig. 2. Quantum yield of photosystem II electron transport (∆F\FMh) measured at different photosynthetic photon fluxes (PPF) in leaves of potato plantlets cultured in the absence (open circles) or presence (closed circles) of the mycorrhizal fungus Glomus intraradices under a PPF of 60 (a, b) or 300 (c, d) µmol m−# s−" and under CO concentrations of 350 (a, c) or 10 000 (b, d) ppm. # Printed from the C JO service for personal use only by... Growth and photosynthesis of mycorrhizal potato plantlets 1·0 (a) (b) (c) (d) 547 0·8 Quenching coefficients (qp and qN) 0·6 0·4 0·2 0·0 1·0 0·8 0·6 0·4 0·2 0·0 0 200 400 600 800 1000 0 200 400 600 800 1000 PPF (µmol m–2 s–1) Fig. 3. Values of the photochemical qP (triangles) and non-photochemical qN (squares) quenching coefficients measured at different photosynthetic photon fluxes (PPF) in leaves of potato plantlets cultured in the absence (open symbols) or presence (closed symbols) of the mycorrhizal fungus Glomus intraradices under a PPF of 60 (a, b) or 300 (c, d) µmol m−# s−" and under CO concentrations of 350 (a, c) or 10 000 (b, d) ppm. # estimated by the fluorescence parameter ∆F\Fmh ratio (Genty et al., 1989) in the plantlets grown under low PPF compared with those from high PPF (Figs. 2a,c). These more pronounced decreases of ∆F\Fmh in plantlets grown under low PPF and CO # were associated with larger decreases of the photochemical qP and larger increases of the nonphotochemical qN quenching coefficients as PPF increased (Fig. 3a,c). As observed for the light saturation curves of O # evolution, CO -enrichment had no effect on # fluorescence parameters in plantlets grown at low PPF (Figs. 2a,b ; Fig. 3a,b). However, CO -en# richment had a negative effect on fluorescence parameters in plantlets grown under high PPF. When subjected to increasing PPF, ∆F\Fmh and qP measured from those leaves decreased the most rapidly and qN increased to the maximum value (Figs 2d,3d). These results clearly indicate a limitation of photosynthetic electron transport in leaves developed under these conditions. It is important to note that these negative effects of high PPF and CO # growth levels on ∆F\Fmh and qP were less in mycorrhizal plantlets than in the controls. The differences are larger at PPFs near those during growth (i.e. 300 µmol m−# s−"). Such effects of mycorrhiza on ∆F\Fmh and qP at high PPF and CO , # although limited, are consistent with their effects on Chlajb and carotenoid contents, Φmax and Fv\Fm (Tables 3, 4). In this study, we used the in vitro tripartite culture system (Elmeskaoui et al., 1995) as a simple model to determine the importance of two environmental factors, CO and PPF, on the mycorrhizal association # between G. intraradices and potato vitroplants via their effects on the plant source–sink relationship. Our results demonstrated that CO enrichment # (from 350 to 10 000 ppm) during the tripartite culture resulted in an increased percentage of mycorrhizal infection in roots of potato vitroplants. These results support those of Elmeskaoui et al. (1995) who observed higher in vitro infection rates of strawberry plantlets by G. intraradices under elevated CO # (5000 ppm) compared with normal CO . The very # high CO concentration used in our experiments is # consistent with other artificial growth systems. It was also used in an in vitro culture system where it Printed from the C JO service for personal use only by... 548 D. Louche-Tessandier et al. stimulated growth of an epiphytic Crassulacean acid metabolism (CAM) orchid (Gouk et al., 1997). In a proposed life support system in space, 10 000 ppm CO had no significant effect on vegetative growth of # wheat but decreased seed yield by 37%, probably because of a partial suppression of respiration required for grain filling (Reuveni & Bugbee, 1997). The stimulation of mycorrhizal infection by high CO concentration during the tripartite culture has # two possible origins : CO enrichment stimulates # photoautotrophic C uptake and consequently increases C allocation to the root system and to the VA symbiont ; high CO concentration acts syner# gistically with root exudates to stimulate VA-hyphal growth and thereby increasing inoculum strength (Chabot et al., 1996 ; Elmeskaoui et al., 1995). Although we cannot clearly determine the relative importance of these two possibilities without further experiments, we suggest that after 4 wk of our experiments, the latter is the most likely for two reasons. First, increased PPF under normal CO # concentration did not increase the percentage of root infection despite large increases of maximal photosynthetic capacity, root : shoot ratio and DM production. By contrast, CO enrichment promoted # mycorrhizal infection under low PPF with no significant effects on maximal photosynthetic capacity, root : shoot ratio and DM production. Therefore, it appears that in our experimental conditions, the stimulation of mycorrhizal colonization by high CO does not result from an increase of photo# synthetic C fixation. We must stress that this conclusion might be different from that reached in experiments carried out in natural conditions where the increased CO concentrations generally do not # exceed the naturally high CO concentration in the # soil atmosphere of c. 1000 ppm (Hodge, 1996). In these experiments, increased C partitioning to the root system is considered as the main cause for the CO -enhancement of mycorrhizal colonization # (Hodge, 1996 ; Rillig et al., 1998). The levels of mycorrhizal infection measured in potato roots after 28 d of experiments were relatively low compared with those reported previously for strawberry plantlets developed in the in vitro tripartite culture system (Herna! ndez Sebastia' , 1998). Our results, and those from previous studies on mycorrhizas with potato plants, suggest that potato is a species showing generally low levels of mycorrhizal colonization but still high morphological and physiological responses to the presence of the mycorrhizal fungus. Niemira et al. (1995) observed yield increases and favourable morphological changes of prenuclear minitubers of potato developed in the presence of very low levels of mature mycorrhizal association. Also, it was shown recently that low levels of mycorrhizal colonization (5–15%) increased resistance of potato plants to pathogens such as Fusarium (Niemira et al., 1996). Our results demonstrate that the CO concen# tration during the tripartite culture affected not only the degree of mycorrhizal colonization in in vitro potato roots but also the impacts of the mycorrhiza on the growth and physiology of potato vitroplants. Under normal CO concentration during the tri# partite culture, no difference could be observed between control and mycorrhizal plantlets in DM production, pigment contents and photosynthetic maximum efficiency and maximum capacity. The only significant difference we noticed under normal CO concentration was a higher stomatal conduc# tance in mycorrhizal plantlets than the controls, except in plantlets grown under high CO and PPF # which showed impaired stomatal function. This stimulation of stomatal conductance is consistent with previous results showing an increase of stomatal conductance and transpiration rate in mycorrhizal plants (Fitter, 1988). Therefore, the in vitro mycorrhization of potato plantlets under normal CO # concentration during the tripartite culture altered plant water relations but did not affect growth and photosynthetic characteristics. This implies that under normal CO concentration, the C cost to the # mycorrhizal fungus was balanced with a small stimulation of in situ photosynthetic rate which could have been undetected during our photosynthetic measurements made under saturating CO # conditions. Recently, Wright et al. (1998) observed a stimulation of photosynthetic rates in mycorrhizal clover plants measured at 330 ppm CO without # enhancement of plant dry weight, suggesting that the additional fixed C was allocated to mycorrhizal fungus. In contrast to normal CO concentration during # the tripartite culture, mycorrhizal colonization under 1% CO had significant effects on the growth and # physiology of potato vitroplants. However, these effects were strongly dependent on the PPF during growth. Under 1% CO and 60 µmol m−# s−", # mycorrhizal plantlets accumulated less DM than non-inoculated plantlets whereas the opposite was observed at 1% CO and 300 µmol m−# s−". These # opposing results can be reconciled by considering the plant source–sink status under both growth conditions. At low PPF, photosynthesis was clearly source-limited since increase of PPF resulted in large increases of DM production. In addition, stimulation under high CO and low PPF of the # mycorrhizal colonization rate and a possible increase of metabolic activity of the mycorrhizal fungi by the elevated CO concentration (Chabot et al., 1992b) # would have increased the total C cost for the infection and therefore the sink strength (Fitter, 1991 ; Hodge, 1996). Consequently, the mycorrhizal relationship induced in our experiments under low PPF and high CO is likely to have caused an imbalance of the # source–sink relationship leading to a C deficit for the host plant. Printed from the C JO service for personal use only by... Growth and photosynthesis of mycorrhizal potato plantlets The situation is quite different in mycorrhizal plantlets grown under both high CO and PPF # which showed a slight increase of DM production compared with non-inoculated plantlets. These high CO and PPF growth levels lead to typical sink # limitation and down regulation of photosynthesis as can be deduced from the following observations. $ The relative increases of DM between 14 and 28 d (j26%) of the experiments under these conditions were low for both mycorrhizal and control plantlets whereas the relative increases observed during this period were much higher for the other growth conditions (66% and 88%). $ The highest values of the R : S ratio were measured in plantlets grown under high CO and PPF. # $ By increasing growth PPF, the maximum efficiency Φmax and maximum capacity Pmax of photosynthetic O evolution as well as the maximum # quantum efficiency of PS II estimated by the chlorophyll fluorescence ratio Fv\Fm all decreased under CO -enriched atmosphere, which is contrary # to normal atmosphere where an increase of growth PPF stimulated Pmax where Φmax and Fv\Fm remained constant. The large decrease of Fv\Fm is a valuable indicator of photoinhibitory damage in these plantlets (Aro et al. 1993). $ Compared with normal CO concentration, in# crease of growth PPF under high CO concentration # resulted in a more pronounced decrease of photosynthetic pigment contents. From these different observations, it is clear that the stimulatory effects of high CO and PPF on growth # of potato plantlets could not be sustained for the whole experiment because of severe sink limitation which resulted in a down regulation of photosynthesis in both mycorrhizal and non-inoculated potato plantlets. A common hypothesis proposed in recent studies related to the impacts of mycorrhizas on plant responses to CO -enrichment is that the mycorrhizal # partner can significantly increase C sink strength and consequently decrease host plant susceptibility to down regulation of photosynthesis associated with the production of large amounts of carbohydrates in source leaves (Hodge, 1996). So far no evidence was found to verify this hypothesis. Lewis & Strain (1996) observed that the response on Pinus taeda to CO -enrichment was not modified by the presence of # the mycorrhizal symbiont and the P supply. More recently Lovelock et al. (1997) reported a positive effect of VA-mycorrhiza on Pmax from leaves of tropical trees developed under elevated CO . How# ever, this effect was attributed to an increase of leaf P content rather than a decrease of sink limitation of photosynthesis since the sucrose and starch contents in leaves were significantly increased in the presence of VA-mycorrhiza. As mentioned already, potato plantlets developed under high CO and PPF in our # 549 experiments clearly showed symptoms of down regulation of photosynthesis. Comparison of growth and photosynthetic parameters from mycorrhizal and non-inoculated plantlets grown under these supra-optimal conditions suggests that mycorrhiza decreased the severity of the symptoms associated with sink limitation and down regulation of photosynthesis. In mycorrhizal plantlets compared with controls we measured higher production of DM, higher contents of Chlajb, higher contents of carotenoids, higher maximum efficiency of O evol# ution (ΦO ) measured at low PPF, higher maximum # quantum yield of PS II photochemistry estimated by the Chla ratio Fv\Fm in dark adapted leaves, and higher quantum yield of photosynthetic electron transport ∆F\Fm as well as higher photochemical quenching coefficient qP when measured at PPF near the growth PPF (300 µmol m−# s−"). All these differences, albeit small, are consistent and tend to demonstrate the potential of mycorrhizal fungi in in vitro tripartite culture to lessen to some extent the feedback inhibition of photosynthesis related to sink limitation in potato plantlets. The effect of mycorrhiza on sink strength may have been reduced in our experiments by the presence of tomato roots in the tripartite culture system. Indeed, the tomato roots growing on exogenous sucrose may have contributed to the C needs of the mycorrhizal fungi, thereby decreasing their dependence on the photosynthetic C uptake by potato vitroplants. The results reported in this study could be relevant for future experiments aiming to optimize growth conditions of mycorrhizal potato plantlets in the tripartite culture. The presence of high levels of both CO and PPF is detrimental in the long term to plant # growth and photosynthetic performances. We suggest that high CO concentrations should be # maintained at least in the first weeks of the triculture in order to stimulate mycorrhizal colonization and the PPF adjusted so that the source capacity matches the sink strength of the plants. From our conclusions, it could be predicted that the optimum growth PPF will be higher for mycorrhizal than non-mycorrhizal plantlets because of the increase of the sink strength through the presence of the mycorrhizal fungus. This work was supported by an operating grant from NSERC awarded to Y. D. and made possible by the Ministry of International Affairs of the Que! bec Government through its scientific and technological cooperation programme with France. Adams WW, Demmig-Adams B, Winter K, Schreiber U. 1990. The ratio of variable to maximum chlorophyll fluorescence from photosystem II, measured in leaves at Printed from the C JO service for personal use only by... 550 D. Louche-Tessandier et al. ambiant temperature and at 77K, as an indicator of the photon yield of photosynthesis. Planta 180 : 166–174. Aro EM, Virgin I, Andersson B. 1993. Photoinhibition of photosystem 2. Inactivation, protein damage and turnover. Biochimica Biophysica Acta 1143 : 113–134. Bowes G. 1991. Growth at elevated CO – photosynthetic # responses mediated through RuBisCO. Plant, Cell and Environment 14 : 795–806. Chabot S, Be! card G, Piche! Y. 1992a. Life cycle of Glomus intraradices in root organ culture. Mycologia 84 : 315–321. Chabot S, Bel-Hid R, Che# nevert R, Piche! Y. 1992b. Hyphal growth promotion in vitro of the VA mycorrhizal fungus, Gigaspora margarita Becker & Hall, by the activity of structurally specific flavonoid compounds under CO -enriched # conditions. New Phytologist 122 : 461–467. Elmeskaoui A, Damont J-P, Poulin M-J, Piche! Y, Desjardins Y. 1995. A tripartite culture system for endomycorrhizal inoculation of micropropagated strawberry plantlets in vitro. Mycorrhizae 5 : 313–319. Farrar JF, Williams ML. 1991. The effects of increased atmospheric carbon dioxide and temperature on carbon partitioning, source–sink relations and respiration. Plant, Cell and Environment 14 : 819–830. Finlay RD, Frostegard A, Sonnerfeldt A-M. 1992. Utilization of organic and inorganic nitrogen sources by ectomycorrhizal fungi in pure culture and in symbiosis with Pinus contorta Dougl. ex Loud. New Phytologist 120 : 105–115. Fitter AH. 1988. Water relations of red clover Trifolium pratense L. as affected by VA mycorrhizal infection and phosphorus supply before and during drought. Journal of Experimental Botany 39 : 595–603. Fitter AH. 1991. Cost and benefits of mycorrhizas – implications for functioning under natural conditions. Experientia 47 : 350–355. Genty B, Briantais J-M, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of fluorescence. Biochimica Biophysica Acta 990 : 87–92. Gerbaud A, Andre! M. 1980. Effect of CO , O and light on # # photosynthesis and photorespiration in wheat. Plant Physiology 66 : 1032–1036. Giovanetti M, Mosse B. 1980. An evaluation of techniques for measuring vesicular–arbuscular mycorrhizal infection in roots. New Phytologist 84 : 489–500. Gouk SS, Yong JWH, Hew CS. 1997. Effects of super-elevated CO on the growth and carboxylating enzymes in an epiphytic # CAM orchid plantlet. Journal of Plant Physiology 151 : 129–136. Herna! ndez Sebastia' C. 1998. Adaptation au stress hydrique des vitroplants de fraisier inoculeT s avec Glomus intraradices Schenk & Smith dans un systeZ me de culture tripartite. PhD thesis, Universite! Laval, Canada. Hodge A. 1996. Impact of elevated CO on mycorrhizal # associations and implications for plant-growth. Biology and Fertility of Soils 23 : 388–398. Krause GH, Weis E. 1991. Chlorophyll fluorescence and photosynthesis – the basics. Annual Review of Plant Physiology and Plant Molecular Biology 42 : 313–349. Lewis JD, Strain BR. 1996. The role of mycorrhizas in the response of Pinus taeda seedlings to elevated CO . New # Phytologist 133 : 431–443. Lichtenthaler HK, Wellburn AR. 1983. Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions 603 : 591–592. Lovelock CE, Kyllo D, Popp M, Isopp H, Virgo A, Winter K. 1997. Symbiotic vesicular – arbuscular mycorrhizal influence maximum rates of photosynthesis in tropical tree seedlings grown under elevated CO . Australian Journal of Plant # Physiology 24 : 185–194. Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologia Plantarum 15 : 473–497. Niemira BA, Hammerschmidt R, Safir GR. 1996. Postharvest suppression of potato dry rot (Fusarium sambucinum) in prenuclear minitubers by arbuscular mycorrhizal fungal inoculum. American Potato Journal 73 : 509–515. Niemira BA, Safir GR, Hammerschmidt R, Bird GW. 1995. Production of prenuclear minitubers of potato with peat-based arbuscular mycorrhizal fungal inoculum. Agronomy Journal 87 : 942–946. Pearson JN, Jakobson I. 1993. The relative contribution of hyphae and roots to phosphorus uptake by arbuscular mycorrhizal plants, measured by dual labelling with $#P and $$P. New Phytologist 124 : 489–494. Perrin R. 1990. Interactions between mycorrhizae and diseases caused by soil-borne fungi. Soil Use Management 6 : 189–195. Reuveni J, Bugbee B. 1997. Very high CO reduces photo# synthesis, dark respiration and yield in wheat. Annals of Botany 80 : 539–546. Rillig M, Allen M, Klironomos J, Field C. 1998. Arbuscular mycorrhizal percent root infection and infection intensity of Bromus hordeaceus grown in elevated atmospheric CO . # Mycologia 90 : 199–205. Schreiber U, Hormann H, Neubauer C, Klughammer C. 1995. Assessment of photosystem II photochemical quantum yield by chlorophyll fluorescence quenching analysis. Australian Journal of Plant Physiology 22 : 209–220. Steel RGD, Torrie JH. 1980. Principles and procedures of statistics. A biometrical approach, 2nd edn. New York, USA : McGraw-Hill. Subramanian KS, Charest C. 1995. Influence of arbuscular mycorrhizae on the metabolism of maize under drought stress. Mycorrhyza 5 : 273–278. van Kooten O, Snel JFH. 1990. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynthesis Research 25 : 147–150. Walker DA. 1989. Automated measurement of leaf photosynthetic O evolution as a function of photon flux density. # Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences 323 : 323–326. Wright D, Scholes J, Read D. 1998. Effects of VA mycorrhizal colonization on photosynthesis and biomass production of Trifolium repens L. Plant, Cell and Environment 21 : 209–216. Printed from the C JO service for personal use only by...