Using TEE in ECMO Deployment: Problems with VV and VA ECMO
advertisement

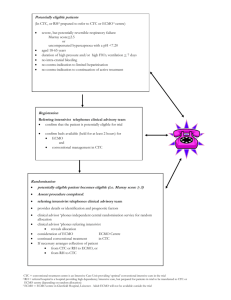
Using TEE in ECMO Deployment: Problems with VV and VA ECMO Robert M. Savage, MD, FACC Vice-Chair, Department of Cardiothoracic Anesthesia & Critical Care Anesthesiology and Heart & Vascular Institutes Cleveland Clinic Healthcare System Cleveland Ohio Objectives Upon completion of this lecture, the participant will have an understanding of the: • Definition, classification, and indication for the different types of ECMO • Role of ECMO in critical care and clinical outcomes • TEE assessment of ECMO deployment, maintainance, & weaning • Role of TEE in troubleshooting ECMO problems & complications ECMO: Definition and Classification Extracorporeal membrane oxygenatin (ECMO) is an advanced strategy increasingly used to support the respiratory and cardiac function of patients with refractory respiratory and/or cardiac failure. ECMO may be used for a few days to support patients with temporarily stunned myocardium with cardiogenic shock or for months in patients with irreversible cardiopulmonary dysfunction as a bridge to heart or lung transplantation. The ECMO circuit consists of a cannula for venous blood drainage, a blood pump, a gas exchange device (oxygenation & CO2 clearance), a thermal control unit, and a cannula for return of oxygenated blood to the patient's venous or systemic arterial system. ECMO is classified as either Veno-Veno (VV) for use in isolated respiratory failure (without RV/LV dysfunction or pulmonary hypertension) or Veno-Arterial (VA) for patients in cardiogenic shock and associated respiratory dysfunction. VV ECMO drains venous blood via a RA or IVC cannula, performs gas exchange, and returns blood to a functioning right heart. VA ECMO uses a similar RA or IVC cannula to drain venous blood which is then oxygenated, cleared of CO2, and returned to the systemic circulation via femoral or central arterial cannulation. ECMO: Role in Critical Care (Indications, Contraindications) & Outcomes The indications for VV ECMO in acute respiratory failure with preserved cardiac function include viral or bacterial pneumonia, SIRS, ARDS, status asthmatacus, traumatic pulmonary contusions, and massive intrapulmonary hemorrhage. VV ECMO may also be used intraoperatively during tracheo-bronchial resections along with jet ventilation. The indications for VA ECMO in cardiogenic shock with associated respiratory dysfunction include "failure to rescue" from cardiopulmonary arrest, cardiogenic shock, inability to wean from CPB, bridge to heart transplant, catastrophic pulmonary embolism, sepsis with myocasrdial depression, myocarditis, and drug overdose with associated cardiac depression. VA ECMO has also been used in the cath lab in higher riskpatients undergoing percutaneous interventions. Contraindications for all forms of ECMO include severe neurologic injury, intracerebral hemorrhage, or chronic irreversible disease in patients not considered candidates for transplantation. VV ECMO is not an option in patients with severe RV or LV dysfunction, severe PHTN, or following cardiopulmonary arrest. Contraindications for VA ECMO are limited to patients with untreated aortic dissections or severe aortic regurgitation. With growth of VA and VV ECMO, an increasing number of centers sre reporting their clinical experience and outcomes. Mohr et al reported a series of 219 consecutive patients with postcardiotomy cardiogenic shock over a 5 year period. The mean duration of VA ECMO was 2.8 days with 134 patients (60%) successfully weaned and 52 (24%) discharged from the hospital at 30 days. Cheng et al performed a metaanalysis of 1529 patients receiving VA ECMO for cardiogenic shock with a cummulative survival-to-discharge of 534 (35%). UPMC reported a series of patients with VV or VA ECMO for PHTN and RV dysfunction with a 30 day survival of 34%. Mason et al described a series of 51 patients with VV ECMO as a bridge to lung transplant with a 2 year survival of 45%. UPMC reported a similar group of 44 patients using ECMO as a bridge to LTP. Though 11 patients (25%) expired before transplant, 33 (75%) were transplanted with 25 (76%) surviving beyond 2 years. TEE Assessment: ECMO Deployment, Maintainance, & Weaning TEE plays an integral role in guiding the management of patients undergoing ECMO imcluding (1)cannulation and ECMO initiation, (2) monitoring during ECMO, (3) ECMO troubleshooting and detection of complications, and (4) weaning from ECMO. Prior to ECMO deployment, a thorough TEE is essential for identifying reversible causes of clinical deterioration (cardiac tamponade, hypovolemia, RV or LV dysfunction, under-diagnosed valve dysfunction), presence of contraindications for ECMO (aortic dissection or severe AR) or issues interfering with correct pls placement of cannula, or ECMO function (ASD, PFO, Chiari Network, prominent Eustachial valve, persistent leftisided SVC, RA thrombi, or TV Stenosis). The pre-ECMO TEE may assist in guiding the choice of ECMO to a VA configuration with the presence of significant RV dysfunction, pulmonary HTN, TR, or acute PE. The presence of severe LV dysfunction, MR, or greater than mild AR may indicate the need for an LV vent to avoid LV distension or a percutaneous bicaval venous cannula to ensure complete emptying of the RV. During cannula insertion for VV ECMO, TEE is useful in guiding the positioning of the venous inflow cannula to the IVC proximal to the RA. The oxygenated ouitflow cannula is positioned in the RA with flow directed across the TV. TEE ensures that neither cannula is positioned in the coronary sinus or across a PFOor ASD. If the dual VV Avulon right internal jugular cannula is used, TEE is used to position the distal tip at the cavoatrial junction with the oxygenated outflow port directed across the tricuspid valve. Precise positioning of this cannula avoids the potential of recirculatimg oxygenated blood into the venous outflow cannula. With TEE's superior image quality, it's more reliable than TTE for positioning the venous cannula in its correct location. Chest x-ray is not real-time or accurate enough to guide positioning of the cannula. In VA ECMO, TEE identification of the guide wire in the aorta before dilitation of the femoral artery ensures the arterial cannula will not be placed retroperitoneally or into the wall of the aorta leading to a retrograde aortic dissection. TEE Troubleshooting: Problems & Complications • Ventricular distension & pulmonary congestion: Because of increased aortic afterload with femoral arterial inflow, TEE monitoring is essential when initiating peripheral fem-fem VA ECMO to ensure aortic valve opening and the absence of LV distension. If the LV distends on initiation of VA ECMO, immediate decompression with a LV vent or atrial baloon septostomy, is necessary to avoid distending the LV with development of severe MR, LA distension, and florrid pulmonary edema. Failure of the aortic valve to open with VA ECMO predisposes thrombus formation on aortic valve cusps necessitating additional anticoagulation. Caunnula malposition or migration & recirculation: With right internal jugular insertion of the single dual lumen cannula for VV ECMO, the tip is positioned above the IVC-RA junction with care to avoid obstruction of the hepatic vein at its insertion into the IVC. Oxygenated blood flow from the outflow lumen must be precisely directed across the TV to avoid hypoxemia related to recirculation of oxygenated blood into the venous inflow cannula. Migration of the cannula with minimal patient repositioning or movement may reduce the distance between the venous inflow & outflow cannula resulting in similar recirculation. Interrogation of this area with CFD or PWD will detect recirculation. Recirculation of oxygenated blood into the inflow cannula brightens the color of blood in the lumen to a shade similar to the oxygenated venous outflow cannula. Budd-Chiari Syndrome: Positioning the femoral venous drainage cannula in the IVC may obstruct from the hepatic vein into the IVC with increased flow velocities (> 1.5 m/sec). This obstruction reduces hepatic venous drainage with hepatic congestion and associated abnormalities in liver enzymes. TEE positioning of cannula below the hepatic vein-IVC insertion relieves obstruction and any associated hepatic congestion. RV rupture & tamponade: When using the dual lumen single venous cannula, the tip may migrate across the TV causing increased TR. With further migration, trumatic rupture of the RV free wall has been reported. Upper Body Hypoxemia Syndrome: In setting of peripheral fem-fem VA ECMO with pulmonary dysfunction with poor oxygenation, incomplete emptying of the RV increases the volume of desaturated blood flowing into the left heart. The enhanced preload increases cardiac output with desaturated blood which impeeds flow of oxygenated blood up the aorta from the femoral artery. With more deoxygenated blood flowing to upper body and head, patient develops a bluish discoloration of head while lower extremities appear pink. This distinct coloration is referred to as the "Upper Body Hypoxemia", "North-South", or "Harlequin" Syndrome. Thrombosis (spontaneous contrast): With prolonged VA ECMO, low or stagnant flow evidenced by spontaneous contrast leads to formation of clot on the cannula andvalve structures. Adjusting ECMO flows, increasing anticoagulation, or decompressing a distended LA or LV may decrease propensity for clot formation. ECMO Weaning VV ECMO: Weaning of VV ECMO is guided largely by changes in oxygenation and pulmonary compliance. Successful weaning is probable if oxygenation and PA pressures do not deteriorate as ECMO flow is progressively decreased by 0.5 L/min flows over 5 minute intervals. CWD interrogation of a TR jet enables estimation of the PA systolic pressure. Marked elevation of PA pressures during VV ECMO weaning may result in RV dilitation, diminished global RV function, and increasing severity of TR. VA ECMO: TEE is more consistently used to guide the weaning process in VA ECMO. TEE assessment of LV Ejection Fraction, LVEDD, severity of MR, and calculated LV stroke volume during progressive 0.5 lpm reductions of VA ECMO circuit flows guides the weaning process over successive 5 minute intervals. Caution must be taken to not reduce ECMO flows below 1 lpm for prolonged periods without increasing risk of clot formation in the circuit. Conclusion: Transesophageal Echocardiography is the quintesential diagnostic imaging technology guiding physicians through the minefield of critical decisions made for patients requiring ECMO. Compared to TTE and other sophisticated fixed imaging techniques, TEE provides a higher resolution of 2&3D imaging and CFD, inaddition to accurate quantitative Doppler capabilities. Because of it's ready availability, portability, cost-effectiveness, and easy to use, TEE it is the navigator of choice providing crucial information for the period surrounding ECMO deployment including: 1) selection of patients, 2) recognition of reversible causes of deterioration, 3) determining appropriate ECMO configuration, 4) identifying contraindications, 5) guiding cannula placement & confirming precise location, 6) monitoring the initiation & maintainance of ECMO support, 7) recognition of complications, and 8) guiding the timing & process of weaning. For centers employing VV & VA ECMO treatment strategie's for their patients, the 24-7 availability of critical care specialists with competency in advanced perioperative echocardiography is a mandatory immutable prerequisite to ensue the quality and safety of care provided to their patients. Bibliography 1. 2. 3. 4. 5. 6. 7. 8. 9. Nicolas Doll, MD, Bob Kiaii, MD, Michael Borger, MD, PhD, Thomas Walther, MD, PhD, and Friedrich W. Mohr, MD, PhD, Five-Year Results of 219 Consecutive Patients Treated With Extracorporeal Membrane Oxygenation for Refractory Postoperative Cardiogenic ShockAnn Thorac Surg 2004;77:151–7 Richard Cheng, MD, Rory Hachamovitch, MD, Michelle Kittleson, MD, PhD, Jignesh Patel, MD, PhD, Francisco Arabia, MD, Jaime Moriguchi, MD, Fardad Esmailian, MD, and Babak Azarbal, MD Complications of Extracorporeal Membrane Oxygenation for Treatment of Cardiogenic Shock and Cardiac Arrest: A Meta-Analysis of 1,866 Adult Patients , Ann Thorac Surg 2014;97:610–6 Hirose et al. Hitoshi Hirose, Kentaro Yamane1, Gregary Marhefka and Nicholas Cavarocchi1, Right ventricular rupture and tamponade caused by malposition of the Avalon cannula for venovenous extracorporeal membrane oxygenation, Journal of Cardiothoracic Surgery 2012, 7:36. Ravi R. Thiagarajan, MBBS, MPH, Thomas V. Brogan, MD, Mark A. Scheurer, MD, Peter C. Laussen, MBBS, Peter T. Rycus, MPH, and Susan L. Bratton, MD, MPH , Extracorporeal Membrane Oxygenation to Support Cardiopulmonary Resuscitation in Adults, Ann Thorac Surg 2009;87:778–85) Christian A. Bermudez, MD, Rodolfo V. Rocha, MD, Penny L. Sappington, MD, Yoshiya Toyoda, MD, PhD, Holt N. Murray, MD, and Arthur J. Boujoukos, MD , Initial Experience With Single Cannulation for Venovenous Extracorporeal Oxygenation in Adults , Ann Thorac Surg 2010;90:991–5 Oxygenation Michael S. Firstenberg, MD, and David A. Orsinelli, MD, FASE, ECMO and ECHO: The Evolving Role of Quantitative Echocardiography in the Management of Patients Requiring Extracorporeal Membrane Mabel Chung,1 Ariel L. Shiloh,1 and Anthony Carlese, Monitoring of the Adult Patient on Venoarterial Extracorporeal Membrane Oxygenation Scientific World Journal Volume 2014, Article ID 393258, 10 pages. Cara L. Agerstrand,* Matthew D. Bacchetta, † and Daniel Brodie, ECMO for Adult Respiratory Failure: Current Use and Evolving Applications ASAIO Journal 2014. Manual of Extracorporeal Membrane Oxygenation (ECMO) in the ICU Hardcover by Poonam Malhotra Kapoor (Editor), Jaypee Brothers Medical Pub, New Delhi, India, – October, 2013. 10. Christian A. Bermudez, MD, Rodolfo V. Rocha, MD, and Arthur J. Boujoukos, MD, Initial Experience With Single Cannulation for Venovenous Extracorporeal Oxygenation in Adults, Ann Thorac Surg 2010;90: 991–5