Michaelis-Menton Method Note: It`s possible to try to visually es mate
advertisement
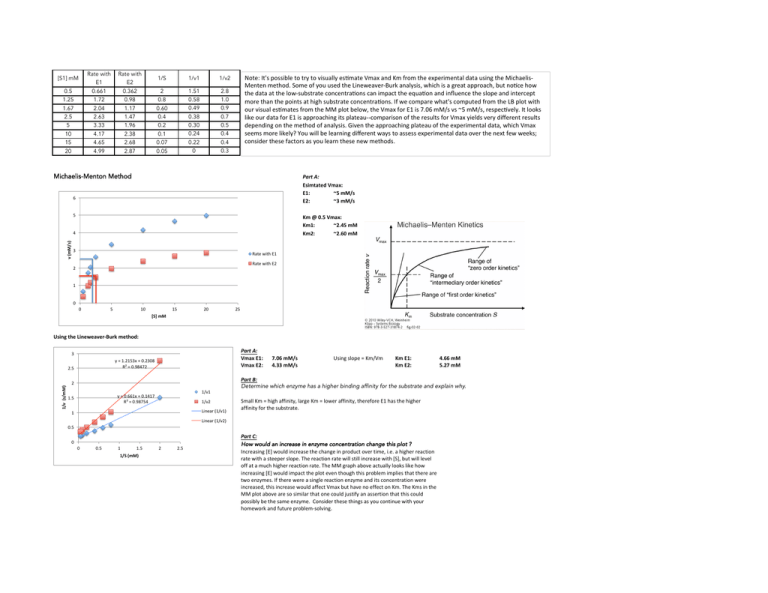
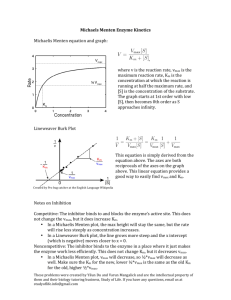
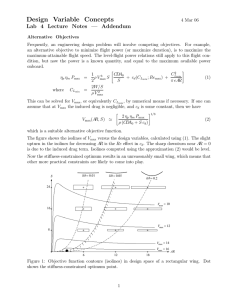
[S1] mM Rate with E1 Rate with E2 1/S 1/v1 1/v2 0.5 1.25 0.661 1.72 0.362 0.98 2 0.8 1.51 0.58 2.8 1.0 1.67 2.5 5 10 2.04 2.63 3.33 4.17 1.17 1.47 1.96 2.38 0.60 0.4 0.2 0.1 0.49 0.38 0.30 0.24 0.9 0.7 0.5 0.4 15 20 4.65 4.99 2.68 2.87 0.07 0.05 0.22 0 0.4 0.3 Note: It's possible to try to visually es^mate Vmax and Km from the experimental data using the Michaelis-­‐ Menten method. Some of you used the Lineweaver-­‐Burk analysis, which is a great approach, but no^ce how the data at the low-­‐substrate concentra^ons can impact the equa^on and influence the slope and intercept more than the points at high substrate concentra^ons. If we compare what's computed from the LB plot with our visual es^mates from the MM plot below, the Vmax for E1 is 7.06 mM/s vs ~5 mM/s, respec^vely. It looks like our data for E1 is approaching its plateau-­‐-­‐comparison of the results for Vmax yields very different results depending on the method of analysis. Given the approaching plateau of the experimental data, which Vmax seems more likely? You will be learning different ways to assess experimental data over the next few weeks; consider these factors as you learn these new methods. Michaelis-Menton Method Part A: Esimtated Vmax: E1: ~5 mM/s E2: ~3 mM/s 6 5 Km @ 0.5 Vmax: Km1: ~2.45 mM Km2: ~2.60 mM v (mM/s) 4 3 Rate with E1 Rate with E2 2 1 0 0 5 10 15 20 25 [S] mM Using the Lineweaver-­‐Burk method: Part A: Vmax E1: Vmax E2: 3 y = 1.2153x + 0.2308 R² = 0.98472 1/v (s/mM) 2.5 2 1/v1 y = 0.661x + 0.1417 R² = 0.98754 1.5 1/v2 Linear (1/v1) 1 7.06 mM/s 4.33 mM/s Using slope = Km/Vm Km E1: Km E2: Part B: Determine which enzyme has a higher binding affinity for the substrate and explain why. Small Km = high affinity, large Km = lower affinity, therefore E1 has the higher affinity for the substrate. Linear (1/v2) 0.5 0 0 0.5 1 1.5 1/S (mM) 2 2.5 4.66 mM 5.27 mM Part C: How would an increase in enzyme concentration change this plot ? Increasing [E] would increase the change in product over time, i.e. a higher reaction rate with a steeper slope. The reaction rate will still increase with [S], but will level off at a much higher reaction rate. The MM graph above actually looks like how increasing [E] would impact the plot even though this problem implies that there are two enzymes. If there were a single reaction enzyme and its concentration were increased, this increase would affect Vmax but have no effect on Km. The Kms in the MM plot above are so similar that one could justify an assertion that this could possibly be the same enzyme. Consider these things as you continue with your homework and future problem-­‐solving.