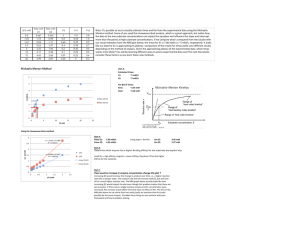
Chapter 14. Enzyme Kinetics Chemical kinetics • • Elementary reactions A → P (Overall stoichiometry) I1 → I2 (Intermediates) Rate equations aA + bB + … +zZ → P Rate = k[A]a[B]b…[Z]z k: rate constant • The order of the reaction (a+b+…+z): Molecularity of the reaction – Unimolecular (first order) reactions: A → P – Bimolecular (second order) reactions: 2A → P or A + B → P – Termolecular (third order) reactions Rates of reactions A → P (First-order reaction) d[P] v= dt =- d[A] dt = k[A] 2A → P (Second-order reaction) v= d[P] dt =- d[A] dt = k[A]2 A + B → P (Second-order reaction) v= d[P] dt =- d[A] dt =- d[B] dt = k[A][B] Rate constant for the first-order reaction d [A] = k[A] dt d [A] = −kdt [A] [ A] d [A] t ∫[A]0 [A] = −k ∫0 dt ln[A] − ln[A]0 = −kt v=− ln[A] = ln[A]0 − kt [A] = [A]0 e − kt The reactant concentration decreases exponentially with time (t) Half-life is constant for a first-order reaction ln[A] − ln[A]0 = −kt [A] ln = −kt [A]0 1 ln = −kt 1 / 2 2 ln 2 0.693 t1 / 2 = = k k For the first-order reaction, half-life is independent of the initial reactant concentration Second-order reaction with one reactant 2A → P d [A] = k [ A ]2 dt d [A] = −kdt 2 [A] [ A] d [A] t k = − ∫[A]0 [A]2 ∫0 dt 1 1 = + kt [A] [A]0 v=− 1 Slope= k [A] 1 [A]0 Time (t) kt 1 / 2 = 2 1 1 − = [A]0 [A]0 [A]0 1 t1 / 2 = Half-life is dependent on the initial reactant concentration k[A]0 Pseudo first-order reactions v = k[A][B] When [B] >> [A], v = k ′[A] where k ′ = k[B]0 e.g. B is water (55.5M): k'= 55.5k Arrhenius equation k = Ae-Ea/RT = Activation energy (Ea) Multistep reactions have rate-determining steps k1 > k2 k1 < k2 k1 k2 Rate-determining step: the slow step (= the higher activation energy) Catalysts reduce the activation energy ΔEa (the reduction in Ea by the catalyst) Rate enhancement = kcat/kuncat = e ΔEa/RT e.g. When ΔEa = 5.7 kJ/mol, the reaction rate increases 10 fold When ΔEa = 34 kJ/mol, the reaction rate increases 106 fold Michaelis-Menten equation Vmax [S] v0 = K M + [S] • • • • v0 = the initial rate Vmax = the maximum rate KM = the Michaelis constant [S] = the substrate concentration Steady state approximation E+S k1 k-1 ES k2 P+E Derivatization of Michaelis-Menten equation k1 E+S ES k2 P+E k-1 v= d [ P] = k 2[ES] dt d [ES] = k 1[E][S] − k − 1[ES] − k 2[ES] = 0 (Steady state approximation) dt [E]T = [E] + [ES] k 1([E]T − [ES])[S] = (k − 1 + k 2)[ES] (k − 1 + k 2 + k 1[S])[ES] = k 1[E]T[S] k − 1 + k2 + [S])[ES] = [E]T[S] k1 [E]T[S] [ES] = k − 1 + k2 + [S] k1 [E]T[S] k − 1 + k2 [ES] = where KM = KM + [S] k1 k 2[E]T[S] The initial velocity v 0 = k 2[ES] = KM + [S] The maximum velocity (Vmax) occurs at high substrate concentration ( when the enzyme is entirely in the [ES] form : Vmax = k 2[E]T v0 = Vmax [S] K M + [S] Michaelis constant KM KM = [S] at which v0 = Vmax/2 • • If an enzyme has a small value of KM, it achieves maximal catalytic efficiency at low substrate concentrations Measure of the enzyme’s binding affinity for the substrate (The lower KM, the higher affinity) Lineweaver-Burke plot Vmax [S] v0 = K M + [S] 1 K M + [S] = v 0 Vmax [S] 1 ⎛ KM ⎞ 1 1 ⎟⎟ = ⎜⎜ + v 0 ⎝ Vmax ⎠ [S] Vmax kcat/KM is a measure of catalytic efficiency Catalytic constant or turnover number of an enzyme : Vmax kcat = [E]T When KM >> [S], very little ES is formed and consequently [E] ≈ [E]T Vmax[S] Vmax[S] kcat[E]T[S] ⎛ kcat ⎞ v0 = ≈ = ≈ ⎜ ⎟[E][S] KM + [S] KM KM ⎝ KM ⎠ Catalytic perfection kcat k 2 k 1k 2 = = KM K M k − 1 + k 2 The ratio is maximal when k 2 >> k − 1, kcat = k1 KM (Diffusion-controlled limit: 108 to 109 M-1s-1) Inhibitors • Substances that reduce an enzyme’s activity – Study of enzymatic mechanism – Therapeutic agents • Reversible or irreversible inhibitors CO2- O O N HN H2N N N N H CO2- O CO2- N Dihydrofolate (Dihydrofolate reductase substrate) N N H H N H NH2 H2N N N CO2- N CH3 Methotrexate (Dihydrofolate reductase inhibitor, anticancer drug ) Modes of the reversible inhibition • Competitive inhibitors – Binds to the substrate binding site • Uncompetitive inhibitors – Binds to enzymesubstrate complex • Non-competitive inhibitors – Binds to a site different from the substrate binding site • Mixed inhibitors – Binds to the substratebinding site and the enzyme-substrate Competitive inhibition KI = [E][I] [EI] d [ES] = k 1[E][S] − k − 1[ES] − k 2[ES] = 0 (Steady state approximation) dt ⎛ k − 1 + k 2 ⎞ [ES] KM [ES] = [E] = ⎜ ⎟ [S] ⎝ k 1 ⎠ [S] [E][I] KM [ES][I] = [EI] = KI [S]KI [E]T = [E] + [EI] + [ES] [E]T = ⎧ KM ⎛ [I] ⎞ ⎫ KM [ES] KM [ES][I] + + [ES] = [ES]⎨ ⎜1 + ⎟ + 1⎬ [S] [S]KI ⎩ [S] ⎝ KI ⎠ ⎭ [E]T[S] ⎛ [I] ⎞ KM ⎜1 + ⎟ + [S] ⎝ KI ⎠ k 2[E]T[S] v 0 = k 2[ES] = ⎛ [I] ⎞ KM ⎜1 + ⎟ + [S] ⎝ KI ⎠ Since Vmax = k 2[E]T, [ES] = v0 = Vmax [S] αK M + [S] where α = 1 + [I] KI Competitive inhibitors affect KM v0 = Vmax [S] αK M + [S] [S] → ∞; v0 → Vmax regardless of α Determination of KI of the competitive inhibitor 1 ⎛ α KM ⎞ 1 1 + =⎜ ⎟ v0 ⎝ V max ⎠ [S] V max Uncompetitive inhibition [ES][I] [ESI] KM [ES] [E] = [S] [ES][I] [ESI] = KI ' [E]T = [E] + [ES] + [ESI] KI ' = [E]T = ⎛ KM [I] ⎞ KM [ES] [ES][I] + [ES] + = [ES]⎜⎜ + 1 + ⎟⎟ KI ' KI ' ⎠ [S] ⎝ [S] [E]T[S] ⎛ [I] ⎞ KM + ⎜1 + ⎟[S] ⎝ KI ' ⎠ k 2[E]T[S] v 0 = k 2[ES] = ⎛ [I] ⎞ KM + ⎜1 + ⎟[S] ⎝ KI ' ⎠ Since Vmax = k 2[E]T, [ES] = Vmax [S] Vmax [S] α ' = v0 = K M + α '[S] K M + [S] α' where α ' = 1 + [I] KI ' Uncompetitive inhibitors decrease both Vmax and KM Vmax [S] ' v0 = α KM + [S] α' Vmax α' V K M >> [S]; v0 → max [S] KM [S] → ∞; v0 → Vmax (Effects of an uncompetitive inhibitor become negligible) no inhibitor (α'=1) Increasing [I] VmaxI v0 Vmax/2 α ' = 1+ VmaxI/2 KMI KM [S] [I] KI ' Determination of KI’ of the uncompetitive inhibitor 1 ⎛ KM ⎞ 1 α' =⎜ + ⎟ v0 ⎝ V max ⎠ [S] V max Mixed inhibition KI = [E][I] [EI] KI ' = [ES][I] [ESI] [E][I] KI [ES][I] [ESI] = KI ' [E]T = [E] + [EI] + [ES] + [ESI] [EI] = ⎛ [I] ⎞ ⎛ [I] ⎞ [E]T = [E]⎜1 + ⎟ + [ES]⎜1 + ⎟ ⎝ KI ' ⎠ ⎝ KI ⎠ [E]T = [E]α + [ES]α ' [E]T = ⎞ ⎛ αKM KM [ES] α + [ES]α ' = [ES]⎜⎜ + α ' ⎟⎟ [S] ⎠ ⎝ [S] [E]T[S] αKM + α '[S] k 2[E]T[S] v 0 = k 2[ES] = αKM + α '[S] Since Vmax = k 2[E]T, [ES] = v0 = Vmax [S] αK M + α '[S] where α = 1 + [I] [I] and α ' = 1 + KI KI ' Lineweaver-Burk plot of a mixed inhibition 1 ⎛ αKM ⎞ 1 α' + =⎜ ⎟ v0 ⎝ V max ⎠ [S] V max Noncompetitive inhibition A special mixed inhibition when KI = KI’ v0 = Vmax [S] αK M + α '[S] where α = 1 + [I] [I] and α ' = 1 + KI KI ' When KI = KI' , α = α ' Vmax [S] Vmax [S] v0 = = α α ( K M + [S]) K M + [S] Noncompetitive inhibitors affect not KM but Vmax Vmax v0 = α [S] K M + [S] where α = 1 + [S] → ∞; v0 → [ I] KI Vmax α Vmax no inhibitor (α=1) Vmax v0 Increasing [I] I Vmax/2 VmaxI/2 α = 1+ KM [S] [I] KI Determination of KI of the noncompetitive inhibitor α 1 ⎛ α KM ⎞ 1 + =⎜ ⎟ v0 ⎝ V max ⎠ [S] V max 1/v0 α=2 α= 1.5 Increasing [I] α= 1 (no inhibitor) Slope=αKM/Vmax α/Vmax − 1 KM 0 α = 1+ [ I] KI 1/[S] Effects of inhibitors on Vmax and KM of the Michaelis-Menten equation app Vmax [S] v0 = app K M + [S] Effects of pH • Binding of substrate to enzyme • Catalytic activity of enzyme ' Vmax = Vmax / f 2 and K M' = K M ( f 1 / f 2) • Ionization of substrate where KE2 [H + ] f1= +1+ + KE1 [H ] • Variation of protein structure (only at extreme pHs) EH+ EH + S KE1 KES2 [H + ] f2= +1+ + KES1 [H ] ES- KE2 H+ EH2+ KES2 k1 k-1 H+ ESH KES1 H+ ESH2+ ' Vmax [S] v0 = ' K M + [S] k2 P + EH Approximate identity of catalytic amino acid residues pKa ~4 → Catalytic Asp or Glu residue pKa ~6 → Catalytic His residue pKa ~10 → Catalytic Lys residue Caution should be taken because pKa of amino acid residues are environmentally sensitive Bisubstrate reactions • 60% of biochemical reactions involve two substrates and two products • Transfer reactions and oxidation-reduction reactions Cleland’s nomenclature system for the enzymatic reactions • Substrates: A, B, C, D… in the order that they add to the enzyme • Products: P, Q, R, S… in the order that they leave the enzyme • Inhibitors: I, J, K, L… • Stable enzyme complexes: E, F, G, H… with E being the free enzyme Numbers of reactants and products: Uni (one), Bi (two), Ter (three), and Quad (four) e.g. Bi Bi reaction: a reaction that requires two substrates and yields two products • Types of Bi Bi Reactions • Sequential reactions (single displacement reactions): all substrates bind before chemical event Ordered mechanism Random mechanism • Ping pong reactions (double displacement reactions): chemistry occurs prior to binding of all substrates Differentiating bisubstrate mechanisms • Measure rates • Change concentration of substrates and products • Lineweaver-Burk plot – Intercept (1/Vmax): the velocity at saturated substrate concentration → It changes when the substrate A binds to a different enzyme form with the substrate B – Slope (KM/Vmax): the rate at low substrate concentration → It changes when both A and B reversibly bind to an enzyme form Ping Pong Bi Bi Mechanism • Intercept changes because A and B bind to the different enzyme forms E and F, respectively • Slope remains same because the binding of A and B is irreversible due to the release of the product (P) Sequential Bi Bi Mechanism or Ordered Random • Intercept changes because A and B binds to the different enzyme forms (E or EB) and (EA or E), respectively • Slope changes because the binding of A and B is reversible Differentiating Bi Bi mechanisms by product inhibition Competitive inhibition → Substrate and inhibitor competitively bind to the same site of the enzyme Ping Pong Ordered sequential Random sequential A vs Q: Competitive B vs P: Competitive A vs P: Noncompetitive B vs Q: Noncompetitive A vs Q: Competitive B vs P: Noncompetitive A vs P: Noncompetitive B vs Q: Noncompetitive Under assumption of dead-end complex formation (A is similar with Q and B is similar with P) A vs Q: Competitive B vs P: Competitive A vs P: Noncompetitive B vs Q: Noncompetitive Dead-end complexes A B E Q P Q B A P Dead-end complex (no chemistry) no chemistry competitive ATP + Creatine ADP + Creatine-phosphate competitive Differentiating Bi Bi mechanisms by isotope exchange Ping Pong Mechanism A*→ P isotope exchange is possible without B B* → Q isotope exchange is possible without A or Sequential Mechanism Two substrates are required for the isotope exchange Isotope exchanges in a ping pong mechanism Sucrose phosphorylase (Sucrose) Glucose-fructose + phosphate E Glucose-1-phosphate + fructose Isotope exchange experiments Glucose-fructose + fructose* E Glucose-1-phosphate + phosphate* Glucose-fructose + E Glucose-E + phosphate Glucose-fructose* + fructose E Glucose-1-phosphate* + phosphate E E Fructose + Glucose-E Glucose-1-phosphate + E Ping Pong Mechanism (Double displacement) Isotope exchanges in a sequential mechanism Maltose phosphorylase (Maltose) Glucose-glucose + phosphate E Glucose-1-phosphate + glucose Isotope exchange experiments Glucose-glucose + glucose* E Glucose-1-phosphate + phosphate* Glucose-glucose + phosphate* Glucose-1-phosphate + glucose* E E No isotope exchange E No isotope exchange Glucose-1-phosphate* + glucose Glucose-glucose + phosphate Sequential Mechanism (Single displacement)