Balancing Chemical Equations Worksheet
advertisement

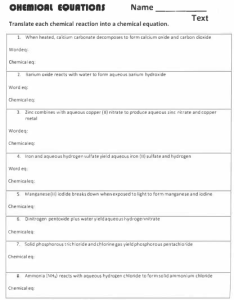
Balancing Equations Worksheet 1) ____ Na3PO4 + ____ KOH ____ NaOH + ____ K3PO4 2) ____ MgF2 + ____ Li2CO3 ____ MgCO3 + ____ LiF 3) ____ P4 + ____ O2 ____ P2O3 4) ____ RbNO3 + ____ BeF2 ____ Be(NO3)2 + ____ RbF 5) ____ AgNO3 + ____ Cu ____ Cu(NO3)2 + ____ Ag 6) ____ CF4 + ____ Br2 ____ CBr4 + ____ F2 7) ____ HCN + ____ CuSO4 ____ H2SO4 + ____ Cu(CN)2 8) ____ GaF3 + ____ Cs ____ CsF + ____ Ga 9) ____ BaS + ____ PtF2 ____ BaF2 + ____ PtS 10) ____ N2 + ____ H2 ____ NH3 11) ____ NaF + ____ Br2 ____ NaBr + ____ F2 12) ____ Pb(OH)2 + ____ HCl ____ H2O + ____ PbCl2 13) ____ AlBr3 + ____ K2SO4 ____ KBr + ____ Al2(SO4)3 14) ____ CH4 + ____ O2 ____ CO2 + ____ H2O 15) ____ Na3PO4 + ____ CaCl2 ____ NaCl + ____ Ca3(PO4)2 16) ____ K + ____ Cl2 ____ KCl 17) ____ Al + ____ HCl ____ H2 + ____ AlCl3 18) ____ N2 + ____ F2 ____ NF3 19) ____ SO2 + ____ Li2Se ____ SSe2 + ____ Li2O 20) ____ NH3 + ____ H2SO4 ____ (NH4)2SO4 Balance these equations! 1) ____ AlBr3 + ____ K ____ KBr + ____ Al 2) ____ FeO + ____ PdF2 ____ FeF2 + ____ PdO 3) ____ P4 + ____ Br2 ____ PBr3 4) ____ LiCl + ____ Br2 ____ LiBr + ____ Cl2 5) ____ PbBr2 + ____ HCl ____ HBr + ____ PbCl2 6) ____ CoBr3 + ____ CaSO4 ____ CaBr2 + ____ Co2(SO4)3 7) ____ Na3P + ____ CaF2 ____ NaF + ____ Ca3P2 8) ____ Mn + ____ HI ____ H2 + ____ MnI3 9) ____ Li3PO4 + ____ NaBr ____ Na3PO4 + ____ LiBr 10) ____ CaF2 + ____ Li2SO4 ____ CaSO4 + ____ LiF 11) ____ HBr + ____ Mg(OH)2 ____ MgBr2 + ____ H2O 12) ____ LiNO3 + ____ CaBr2 ____ Ca(NO3)2 + ____ LiBr 13) ____ AgNO3 + ____ Li ____ LiNO3 + ____ Ag 14) ____ Si(OH)4 + ____ NaBr ____ SiBr4 + ____ NaOH 15) ____ NaCN + ____ CuCO3 ____ Na2CO3 + ____ Cu(CN)2 Chemistry: Chemical Word Equations Directions: Write a balanced chemical equation for each of the word equations below. 1. aqueous sodium chloride reacts with aqueous lead (II) nitrate to yield a lead (II) chloride precipitate and aqueous sodium nitrate 2. aqueous barium nitrate reacts with sulfuric acid [H2SO4(aq)] to yield a barium sulfate precipitate and nitric acid [HNO3(aq)] 3. silver nitrate reacts in solution with potassium chromate to yield a silver chromate precipitate and soluble potassium nitrate 4. solid calcium carbonate reacts with hydrochloric acid [HCl(aq)] to yield aqueous calcium chloride, carbon dioxide gas, and liquid water 5. aqueous zinc chloride reacts with dihydrogen monosulfide gas to yield a zinc sulfide precipitate and hydrochloric acid 6. magnesium nitrate reacts in solution with potassium hydroxide to yield a magnesium hydroxide precipitate and soluble potassium nitrate 7. solid aluminum hydroxide reacts with nitric acid to yield soluble aluminum nitrate and liquid water 8. aqueous lead (IV) nitrate reacts with aqueous sodium sulfate to yield a lead (IV) sulfate precipitate and soluble sodium nitrate 9. aqueous sodium hydroxide reacts with carbon dioxide gas to yield soluble sodium carbonate and liquid water 10. solid magnesium oxide reacts with hydrochloric acid to yield a solution of magnesium chloride and liquid water 11. solid zinc metal reacts with sulfuric acid to yield aqueous zinc sulfate and hydrogen gas 12. solid ferric oxide reacts with solid aluminum metal to yield solid aluminum oxide and solid iron metal